✔ 100% Authentic Product
👁️ Currently Viewing 1578
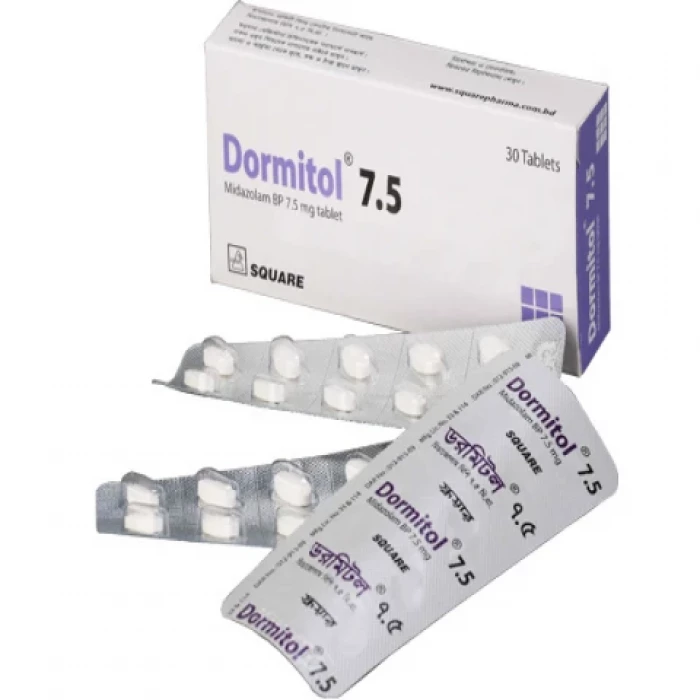
Dormitol 7.5mg 10pcs
Generic Name: Midazolam 7.50mg
Manufacturer/Distributor: Square Pharmaceuticals Ltd.
Discount
Price: ৳ 114
MRP:
৳
120
5%
Off
✅ Description:
Indications
Midazolam is used to treat insomnia for a short period of time.
Before surgical or diagnostic operations, sedation is used as a premedication.
Pharmacology
The inhibitory neurotransmitter gamma-aminobutyric acid (GABA), which is one of the primary inhibitory neurotransmitters in the central nervous system, mediates the effects of benzodiazepines like midazolam. Benzodiazepines enhance GABA activity, causing sedation, relaxation of skeletal muscles, and induction of sleep, anesthesia, and forgetfulness. Benzodiazepines bind to the benzodiazepine site on GABA-A receptors, increasing the frequency of chloride channel opening and therefore potentiating the effects of GABA. These receptors have been found in a variety of bodily tissues, including the heart and skeletal muscle, although they appear to be primarily found in the central nervous system.
Dosage & Administration
Oral dosage:
For adults: 7.5-15 mg daily.
In elderly and debilitated patients: The recommended dose is 7.5 mg.
In premedication: 15 mg of Midazolam should be given 30-60 minutes before the procedure.
Intravenous administration:
Endoscopic or Cardiovascular Procedures: In healthy adults, the initial dose is approximately 2.5 mg. In cases of severe illness and in elderly patients, the initial dose must be reduced to 1 to 1.5 mg.
Induction of Anesthesia: The dose is 10-15 mg.
Intramuscular administration:
Adult: 0.07-0.1 mg/kg body weight. The usual dose is about 5 mg.
Children: 0.15-0.20 mg/kg
Elderly and debilitated patients: 0.025-0.05 mg/kg
Rectal administration in children:
For preoperative sedation: Rectal administration of the ampoule solution (0.35-0.45 mg/kg) 20-30 min. before induction of general anesthesia.
Interaction
Neuroleptics, tranquilizers, antidepressants, sleep-inducing medicines, analgesics, anesthetics, antipsychotics, anxiolytics, antiepileptic drugs, and sedative antihistamines can all be enhanced by midazolam.
Contraindications
Patients with severe respiratory insufficiency, severe hepatic insufficiency, myasthenia gravis, sleep apnea syndrome, or known hypersensitivity to benzodiazepines or any component of the medication should not be administered midazolam.
Side Effect
Drowsiness throughout the day, disorientation, tiredness, headache, and muscular weakness may occur at the start of treatment, although these side effects generally fade away with repeated dosing. Changes in vital signs have been seen following the parenteral (IV or IM) injection of Midazolam, including respiratory depression, apnea, blood pressure alterations, and pulse rate variations.
Pregnancy & Lactation
Unless there is no other option, midazolam should be avoided during pregnancy. Because midazolam travels into breast milk, it should not be given to nursing women.
Precautions & Warnings
Midazolam IV should be administered very slowly.
Storage Conditions
Store in a cool, dry area away from light and moisture. Keep out of children's reach.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.