✔ 100% Authentic Product
👁️ Currently Viewing 1620
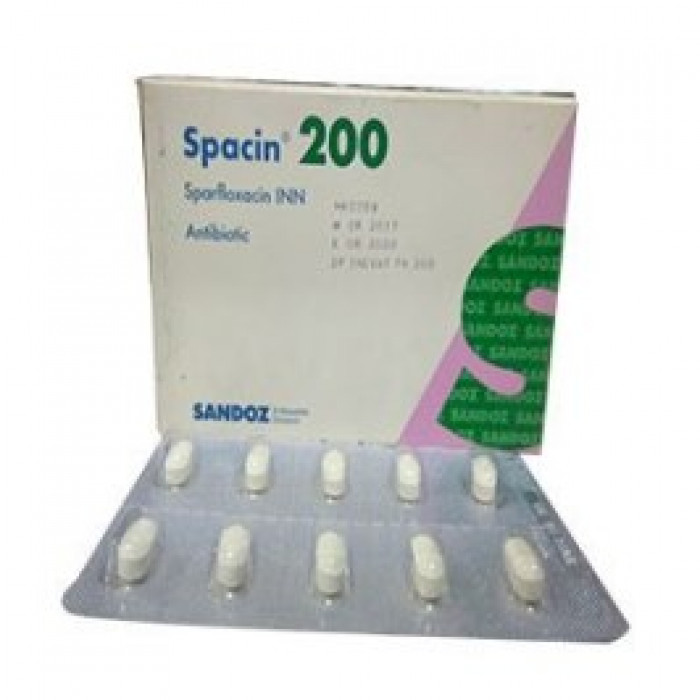
Type: Tab. Generic Name:Sparfloxacin INN 200mg/tablet. Manufacturer/Distributor: Sandoz/Novartis
Discount
Price: ৳ 247
MRP:
৳
260
5%
Off
✅ Description:
Indications
The antibiotic sparfloxacin is used to treat the following infections caused by susceptible microorganisms:
- Sinusitis, acute exacerbation of chronic bronchitis, and community and hospital-acquired pneumonia are examples of upper and lower respiratory tract infections.
- Gonococcal and non-gonococcal urethritis, chancroid, and other sexually transmitted illnesses are all examples of urinary tract infections.
- Infections affecting the skin and soft tissues.
- Use as a prophylactic in a variety of urological and ophthalmology procedures.
Pharmacology
Sparfloxacin is a difluoroquinolone-family synthetic broad-spectrum antibacterial agent. It has been shown to be more active in vitro against several bacteria, notably staphylococci and Mycobacteria, and to have a substantially longer plasma half-life than ciprofloxacin (16 hours).
Sparfloxacin inhibits the supercoiling activity of DNA gyrase, an enzyme required for DNA replication, causing DNA structures to break down. S. pneumoniae, S. aureus, H. influenzae, K. pneumoniae, M. catarrhalis, and Mycobacterium spp. are all susceptible.
Dosage & Administration
In patients with normal renal function the recommended daily dose is two tablets of Sparfloxacin 200 mg on first day as a loading dose, thereafter take one tablet of Sparfloxacin 200 mg every 24 hours for a total of 10 days of therapy.
The recommended daily dose of Sparfloxacin in patients with renal impairment (Creatinine clearance < 30 ml/min) is two tablets of 200 mg taken on the first day as a loading dose. Thereafter, should be taken one tablet of 200 mg every 48 hours for total of 9 days of therapy.
Sparfloxacin can be taken with or without food.
Interaction
On concomitant use with Quinidine, Sotalol, Erythromycin, Astemizole, Terfenadine, vinca alkaloids there is increased risk of arrhythmia. Salts, oxides and hydroxides of Magnesium, Aluminium and Calcium decrease absorption of Sparfloxacin.
Contraindications
Sparfloxacin is contraindicated for individuals with a history of hypersensifivity and in achilles tend in its following the use of fluoroquinolone and in pregnancy and lactation. Sparfloxacin is contraindicated in patients with known QTc prolongation or in patients being treated concomitantly with medications known to produce an increase in the QTc interval and/or torsade depointes.
Side Effects
The majority of the adverse reactions were minor to moderate in severity and only lasted a short time. Diarrhea, nausea, headache, dyspepsia, dizziness, sleeplessness, abdominal discomfort, and QTc interval prolongation are the most commonly reported effects among Sparfloxacin-treated individuals using the recommended dosage.
Pregnancy & Lactation
In pregnant women, there are no sufficient and well-controlled trials. Only if the possible benefit outweighs the risk to the fetus should sparfloxacin be used during pregnancy.
Precautions & Warnings
It should be used with caution in patients with renal disease, stomach ulcers, or who are taking NSAIDs at the same time. Dosage modification is advised in third-degree renal failure (creatinine clearance 30 ml/min): 400 mg on the first day, 200 mg on the second and third days, and then 200 mg every 48 hours. Sparfloxacin should be stopped at the first sign of tendon soreness because fluoroquinolones have been linked to tendon rupture. During therapy, avoid being exposed to UV radiation.
Therapeutic Class
4-Quinolone preparations
Storage Conditions
Store at temperature below 30° C.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.