✔ 100% Authentic Product
👁️ Currently Viewing 4548
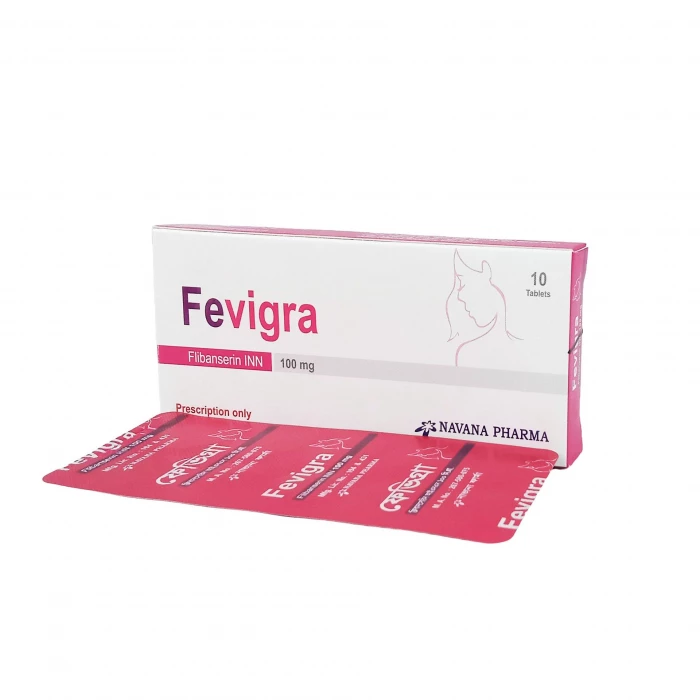
Fevigra 100mg Tablet 10pcs
Flibanserin is indicated for premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD). This condition is characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not caused by a coexisting medical or psychiatric condition, problems within the relationship, or the effects of medication or other drug substances.
Discount
Price: ৳ 475
MRP:
৳
500
5%
Off
✅ Description:
Fevigra is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD). This condition is characterized by low sexual desire that causes marked distress or interpersonal difficulty and is not due to a co-existing medical or psychiatric condition, problems within the relationship, or the effects of medication or other drug substances. Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire, and generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation, or partner.
The recommended dosage of Fevigra is 100 mg orally once per day at bedtime. It's administered at bedtime to mitigate the risks of hypotension, syncope, accidental injury, and central nervous system (CNS) depression. If a dose is missed at bedtime, the next dose should be taken at bedtime the following day without doubling.
Special precautions should be taken when coadministering flibanserin with alcohol or moderate to strong CYP3A4 inhibitors due to the risk of severe hypotension and syncope. CNS depression, including somnolence and sedation, can occur, especially if taken during waking hours or with alcohol or other CNS depressants. Breastfeeding is not recommended during treatment due to potential serious adverse reactions in the breastfed infant.
Safety Advices
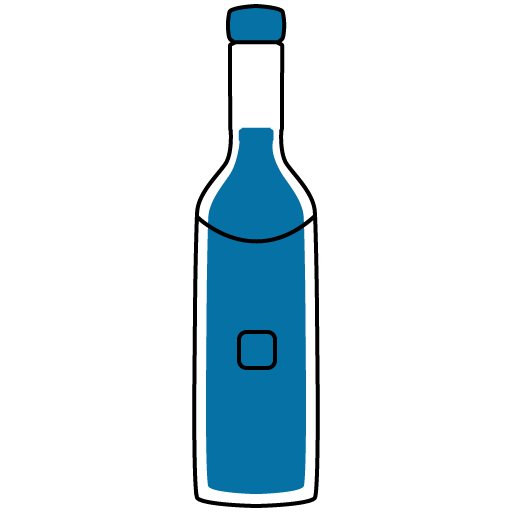
Alcohol
UNSAFE
Drinking alcohol with this medicine can cause dangerous or unwanted side effects.
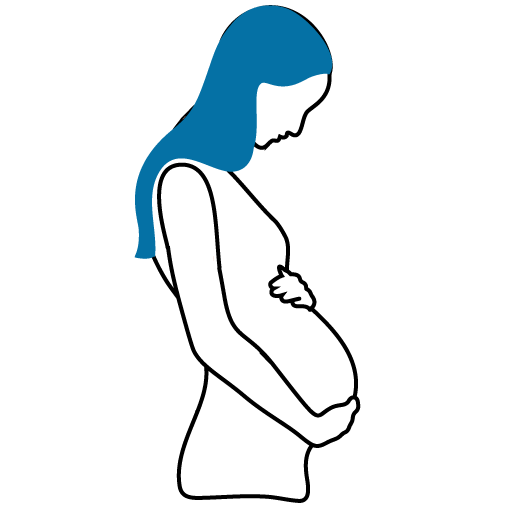
Pregnancy
CONSULT YOUR DOCTOR
There are no adequate studies in pregnant women. Animal studies have shown fetal toxicity only in the presence of significant maternal toxicity.

Breastfeeding
CONSULT YOUR DOCTOR
Flibanserin is not recommended during lactation due to potential adverse effects on the breastfed infant.
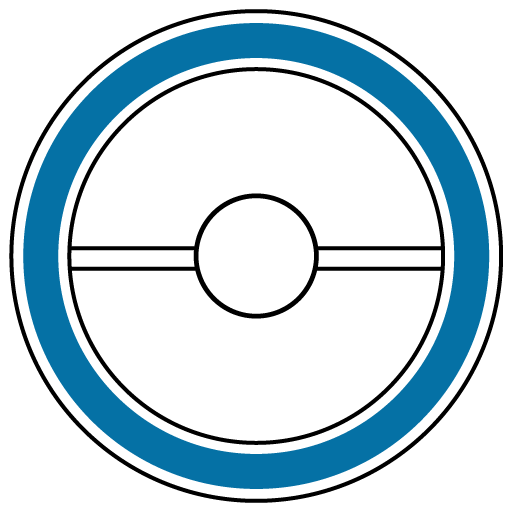
Driving
UNSAFE
Do not drive if you experience any symptoms that affect your ability to concentrate and react.

Kidney
CAUTION
Flibanserin should be used with caution in patients with kidney disease. A dose adjustment of Flibanserin may be needed. Please consult your doctor.
Liver
CAUTION
Flibanserin should be used with caution in patients with severe liver disease. A dose adjustment of Flibanserin may be needed. Please consult your doctor.
✔️ Quick Suggestions of Fevigra 100
- Take Fevigra 100 exactly as prescribed by your doctor. Follow all directions on your prescription label and read all medication guides or instruction sheets.
- Take Fevigra 100 only at bedtime.
- Avoid drinking alcohol for at least 2 hours before taking Fevigra 100 and until the next day. Alcohol may cause you to have dangerously low blood pressure if taken with flibanserin.
- Skip your bedtime dose if you have consumed alcohol less than 2 hours earlier.
- Flibanserin can lower your blood pressure, which can make you dizzy. If you feel light-headed after taking this medicine, lie down if you are not already in bed.
- It may take up to 8 weeks before your symptoms improve. Keep using the medication as directed and tell your doctor if your symptoms do not improve.
✔️ Uses of Fevigra 100
Treats decreased sexual desire in women
✔️ Side Effects of Fevigra 100
Common side effects (>10%) include dizziness, somnolence, and nausea. Other side effects (1-10%) may include fatigue, insomnia, dry mouth, anxiety, constipation, abdominal pain, metrorrhagia, rash, sedation, and vertigo.
✔️ How does Fevigra 100 work?
Flibanserin acts as a postsynaptic serotonin-1A (5HT-1A) receptor agonist and 5HT-2A receptor antagonist. It also exhibits moderate antagonist activities at the 5-HT2B, 5-HT2C, and dopamine D4 receptors. The mechanism of action in HSDD is not fully understood but may involve restoring prefrontal cortex control over the brain's motivation/reward structures, thereby enabling sexual desire to manifest.
✔️ Pharmacology:
Flibanserin, the active ingredient in Fevigra, has a high affinity for serotonin receptors in the brain, acting as an agonist on 5-HT1A and an antagonist on 5-HT2A receptors. It may also moderately antagonize D4 (dopamine) receptors and 5-HT2B and 5-HT2C receptors. Its action on neurotransmitter receptors may contribute to a reduction in serotonin levels and an increase in dopamine and norepinephrine levels, all of which may play a part in reward processing.
✔️ Adult Dose:
For hypoactive sexual desire disorder, the recommended dose is 100 mg orally once daily at bedtime. It's dosed at bedtime to mitigate risks of hypotension, syncope, accidental injury, and CNS depression. Treatment should be discontinued after 8 weeks if no improvement is observed.
✔️ Interaction:
Fevigra may interact with oral contraceptives and other weak CYP3A4 inhibitors, strong CYP2C19 inhibitors, CYP3A4 inducers, and digoxin.
✔️ Contraindications:
Fevigra is contraindicated in patients using alcohol, concomitant use with moderate or strong CYP3A4 inhibitors, and in patients with hepatic impairment.
✔️ Pregnancy & Lactation:
There are no studies of Fevigra in pregnant women. It's excreted in rat milk, and its presence in human milk and effects on the breastfed infant are unknown. Breastfeeding is not recommended during treatment with Fevigra.
✔️ Precautions & Warnings:
Patients experiencing pre-syncope should immediately lie supine and seek medical help if symptoms persist. CNS depression can occur with Fevigra alone and may be exacerbated by other CNS depressants. Patients should avoid activities requiring full alertness until they know how Fevigra affects them.
✔️ Storage Conditions:
Keep below 30°C temperature, away from light & moisture. Keep out of the reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.








