✔ 100% Authentic Product
👁️ Currently Viewing 4613
Human Insulin treats type 1 and type 2 diabetes mellitus, a condition with elevated glucose levels.
Discount
Price: ৳ 539
MRP:
৳
550
2%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
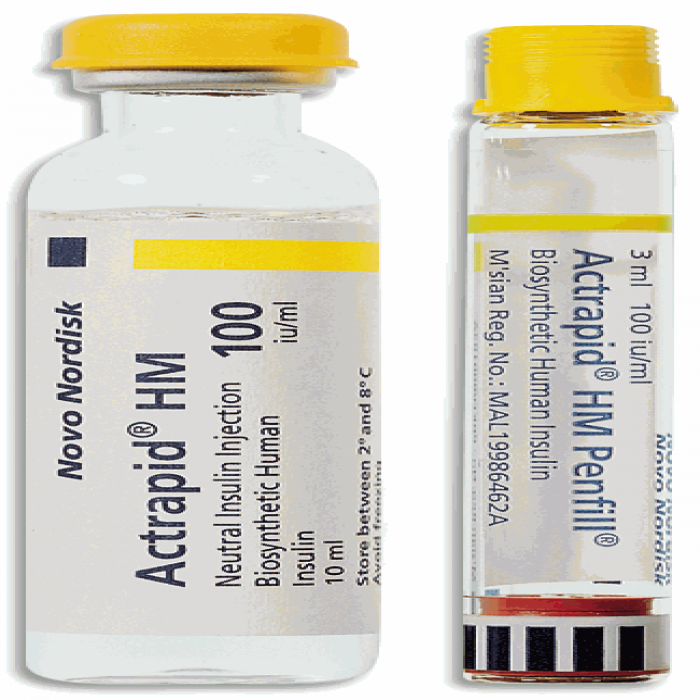
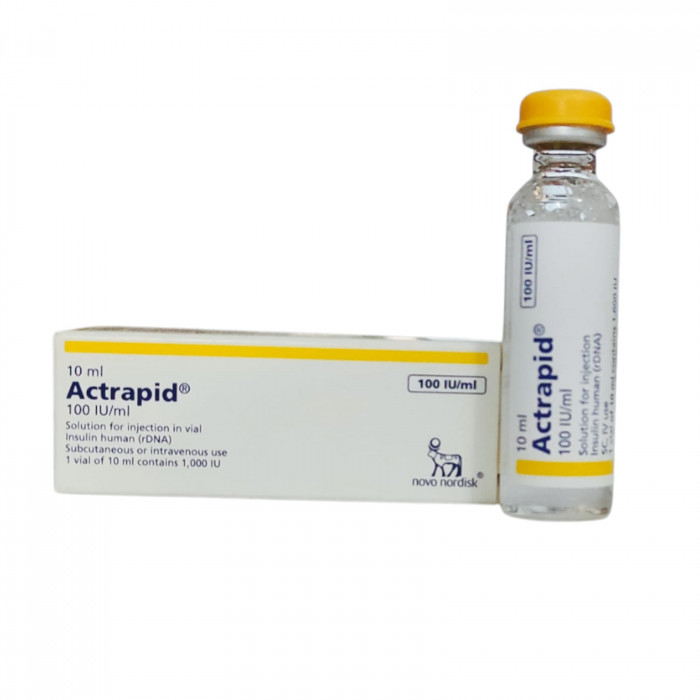
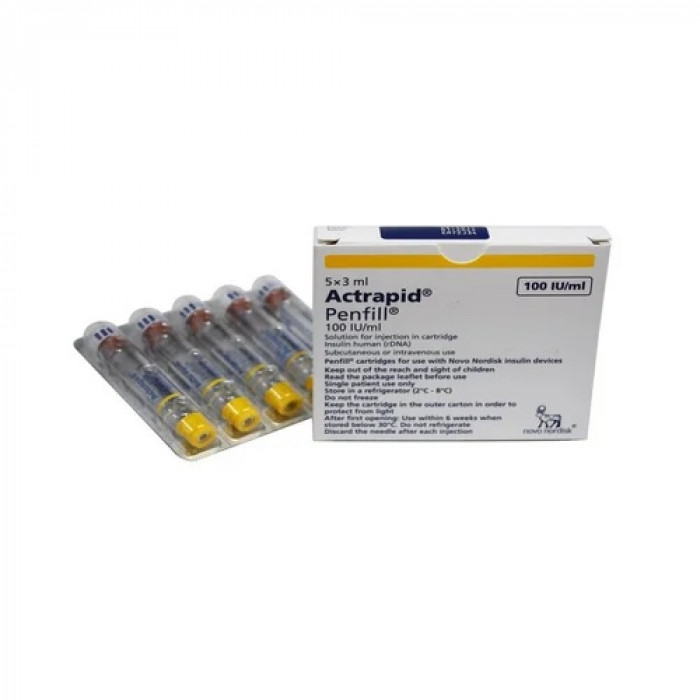
Actrapid 100IU/ml Flexpen 3ml is classified as an 'antidiabetic' medication, serving to treat both type 1 and type 2 diabetes mellitus. This condition involves elevated glucose levels in the body. In type 1 diabetes, insufficient insulin is produced, while in type 2 diabetes, either insulin production decreases or the body becomes resistant to its effects.
Containing 'Human Insulin', Actrapid 100IU/ml Flexpen 3 ml acts as a replacement for the lacking insulin in diabetic patients. By curbing liver sugar production and enhancing sugar uptake by muscle and fat cells, it ensures prompt and consistent sugar control. This insulin commences its action within 30 minutes post-injection and remains effective for up to 8 hours.
Follow your doctor's instructions when taking Actrapid 100IU/ml Flexpen 3 ml. You will be guided by a healthcare professional on the proper injection technique. While the medication can lead to side effects like low blood sugar, hand or foot swelling, weight gain, and skin thickening at injection sites, consult your doctor if these effects persist or worsen.
Avoid using Actrapid 100IU/ml Flexpen 3 ml if you have insulin allergies or a history of hypoglycemia (low blood sugar). Discuss with your doctor if you have liver or kidney disease, or if you are pregnant or breastfeeding. Additionally, inform your doctor about other antidiabetic medications you may be taking. Seek immediate medical attention in case of hypoglycemia.
Safety Advices

Alcohol
UNSAFE
Avoid alcohol consumption as it can worsen side effects and increase the risk of low blood sugar levels.

Pregnancy
CONSULT YOUR DOCTOR
Use Actrapid 100IU/ml Flexpen during pregnancy only if the benefits outweigh the risks. Consult your doctor before starting if pregnant or planning to conceive.

Breastfeeding
CONSULT YOUR DOCTOR
Use Actrapid 100IU/ml Flexpen in breastfeeding mothers only if clinically needed.

Driving
CAUTION
Driving may be affected by high or low blood sugar levels. Avoid driving or operating machinery if experiencing blurred vision, dizziness, or drowsiness due to extreme blood sugar levels.

Kidney
CONSULT YOUR DOCTOR
Use Actrapid 100IU/ml Flexpen cautiously in patients with kidney diseases. Dosage adjustments may be needed.

Liver
CONSULT YOUR DOCTOR
Use Actrapid 100IU/ml Flexpen cautiously in patients with liver diseases. Dosage adjustments may be needed.
✔️ Uses of Actrapid 100IU/ml Flexpen
- Type 1 and Type 2 Diabetes Mellitus.
✔️ How does Actrapid 100IU/ml Flexpen work?
Actrapid 100IU/ml Flexpen assists in optimizing the utilization of circulating blood glucose. This medication facilitates the transportation of glucose from the bloodstream into muscle and fat tissues. Moreover, it curtails the liver's glucose production. Consequently, it effectively regulates blood sugar levels and enhances glycemic control in individuals with diabetes mellitus.
✔️ Side Effects of Actrapid 100IU/ml Flexpen
- Low blood sugar
- Swelling in your hands or feet
- Weight gain
- Thickening of the skin at the injection
✔️ Quick Suggestions:
- Incorporate foods rich in healthy carbohydrates, fiber, fruits, whole grains, and vegetables into your diet.
- Maintain regular eating intervals and avoid skipping meals. Avoid overeating as well.
- Follow a balanced diet and engage in regular walks to complement your Actrapid 100IU/ml Flexpen 3ml treatment.
- Aim for a healthy weight by engaging in regular exercise.
- Prioritize proper rest and manage stress through practices like meditation or yoga.
- Administer Actrapid 100IU/ml Flexpen 3ml about 20 to 30 minutes before a meal.
- Hypoglycemia (low blood sugar level) is a common side effect. Learn to recognize and manage its symptoms (sweating, rapid heartbeat, weakness, blurry vision, headache), and educate your family as well.
- Choose the abdomen as the injection site for faster absorption under the skin.
- Rotate injection sites to prevent the development of lumps at a single site.
- Opened vials/cartridges can be stored at room temperature for up to 4 weeks, while unopened vials should be refrigerated (2°C–8°C).
- If you experience symptoms of hypoglycemia (fatigue, dizziness, confusion, heart palpitations, shakiness, and anxiety), seek prompt medical attention.
✔️ Indication
Actrapid 100IU/ml Flexpen 3ml contains Human Insulin, a fast-acting type of insulin. It effectively reduces blood sugar levels in both adults and children. By facilitating the uptake of sugar by muscle and fat cells, it inhibits the liver's sugar production. This action enhances glycemic control, mitigating the potential advancement of diabetes-related complications such as retinopathy, nephropathy, neuropathy, delayed wound healing, diabetic foot ulcers, and more.
✔️ Pharmacology
The blood glucose-lowering effect of insulin occurs by promoting glucose uptake into muscle and fat cells, while simultaneously inhibiting glucose production by the liver. Insulatard is a long-acting insulin with an onset of action within 1½ hours, reaching its maximum effect within 4-12 hours, and lasting for approximately 24 hours.
Insulin has a short half-life in the bloodstream, and its time-action profile is determined solely by its absorption characteristics. This profile is influenced by factors such as insulin dose, injection site, subcutaneous fat thickness, and diabetes type. Insulin pharmacokinetics are therefore subject to significant individual variability.
✔️ Dosage & Administration of Actrapid 100IU/ml Flexpen
Adult Dose:
- Type 1 Diabetes Mellitus:
- Initial: 0.2-0.4 units/kg/day SC divided q8hr or more frequently
- Maintenance: 0.5-1 unit/kg/day SC divided q8hr or more frequently; higher doses may be needed in insulin-resistant patients
- About 50-75% of total daily insulin requirements are given as intermediate- or long-acting insulin, with rapid- or short-acting insulin used before or during meals to meet the remaining balance.
- Type 2 Diabetes Mellitus inadequately controlled by diet, exercise, or oral medication:
- Initial: 0.2-0.4 units/kg/day SC divided q8hr-q12hr
- Intramuscular Diabetic ketoacidosis:
- Initial loading dose of 20 units, followed by 6 units/hr until blood glucose drops to 10 mmol/l, then given 2 hourly.
- Intravenous Diabetic ketoacidosis:
- Given as 1 unit/ml using an infusion pump, starting at 6 units/hr; the rate can be increased if blood glucose doesn't decrease by 5 mmol/l/hr; reduced to 3 units/hr when glucose is around 10 mmol/l.
- Hyperkalemia: 5-10 units IV insulin in 50 mL D50W infused over 15-30 min
- Combination with NPH/Intermediate-acting insulin:
- Morning: Give two-thirds of daily insulin SC, with regular insulin to NPH insulin ratio of 1:2
- Evening: Give one-third of daily insulin SC, with regular insulin to NPH insulin ratio of 1:1
Child Dose:
- Type 1 Diabetes Mellitus:
- Initial: 0.2-0.4 unit/kg/day SC divided q8hr or more frequently
- Maintenance: 0.5-1 unit/kg/day SC divided q8hr or more frequently; higher doses may be needed in insulin-resistant patients
- Adolescents during puberty may require up to 1.5 mg/kg/day
- Prepubertal children: Average total daily insulin requirement varies from 0.7-1 unit/kg/day, possibly lower
Renal Dose:
- Dose adjustments may be needed for renal impairment.
Administration:
- Inject subcutaneously in the abdomen, thigh, or upper arm, rotating injection sites to prevent skin damage.
- Take Actrapid 100IU/ml Flexpen 3 ml 15 minutes before a meal or immediately after food.
Overdose: Excessive Actrapid HM 100IU/ml Penfill use can lead to low blood sugar levels (hypoglycemia) with symptoms like dizziness, fainting, sweating, and tremors. If you have a blood glucose meter, test immediately and confirm. Regardless, consume glucose water/juice or sugary foods and seek medical attention.
Missed Dose: A missed dose might lead to high blood sugar levels with symptoms like thirst, frequent urination, loss of appetite, drowsiness, and fruity breath odor. Take the missed dose as soon as you remember, but if it's close to the next dose, skip it. Avoid doubling the dose, as it can cause a dangerous drop in blood glucose.
✔️ Interaction
Before using Actrapid 100IU/ml Flexpen, it's important to inform your doctor if you are taking any of the following medications:
- Other diabetes medications (e.g., glimepiride, glyburide, gliclazide, rosiglitazone, pioglitazone)
- Asthma medications (e.g., salbutamol, terbutaline)
- Corticosteroids for pain and inflammation (e.g., hydrocortisone, prednisolone)
- Thyroid hormones for thyroid deficiency (e.g., thyroxine, levothyroxine)
- Thiazide diuretics for edema and hypertension (e.g., hydrochlorothiazide, chlorothiazide)
- Pain and inflammation medications (e.g., aspirin)
- High blood pressure medications (e.g., atenolol, propranolol, captopril, enalapril, ramipril, lisinopril, losartan, valsartan, olmesartan, telmisartan)
- Growth hormone supplements
- Medications for diarrhea (octreotide)
- Medications for abnormal body growth (lanreotide)
- Medications for uterine problems (danazol)
- Beta-blockers for heart problems (e.g., atenolol, propranolol, labetalol)
- Antidepressants (moclobemide, isocarboxazid, phenelzine, selegiline, tranylcypromine)
✔️ Contraindications
Avoid using Actrapid 100IU/ml Flexpen if you have an allergy to insulin or any of its components, or if you experience hypoglycemia (low blood sugar levels).
✔️ Pregnancy & Lactation
Before using this medication, it's important to consult your doctor if you are pregnant, suspect pregnancy, or intend to become pregnant. This insulin is considered safe for use during pregnancy. Your insulin dosage may require adjustment during pregnancy and after childbirth. Maintaining proper diabetes management is crucial to prevent hypoglycemia and ensure the well-being of your baby. Breastfeeding is not restricted while using this medication.
✔️ Precautions & Warnings
Do not use Actrapid 100IU/ml Flexpen 3ml if you are allergic to its components. Inform your doctor about conditions like hypoglycemia (low blood glucose levels), hypokalemia (low levels of potassium), heart, kidney, or liver issues. Consult a doctor before using Actrapid 100IU/ml Flexpen 3ml if pregnant or breastfeeding. Be cautious while driving due to possible dizziness. Avoid alcohol consumption to prevent adverse effects. Adjust your insulin schedule if traveling across multiple time zones, as advised by your doctor.
✔️ Storage Conditions
Store Actrapid 100IU/ml Flexpen between 2°C to 8°C in a refrigerator. Do not freeze the medication. Ensure it is kept out of the reach of children. Avoid opening the carton of vials to shield it from light exposure. Unused portions should not be stored and should be disposed of appropriately.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.