✔ 100% Authentic Product
👁️ Currently Viewing 4287
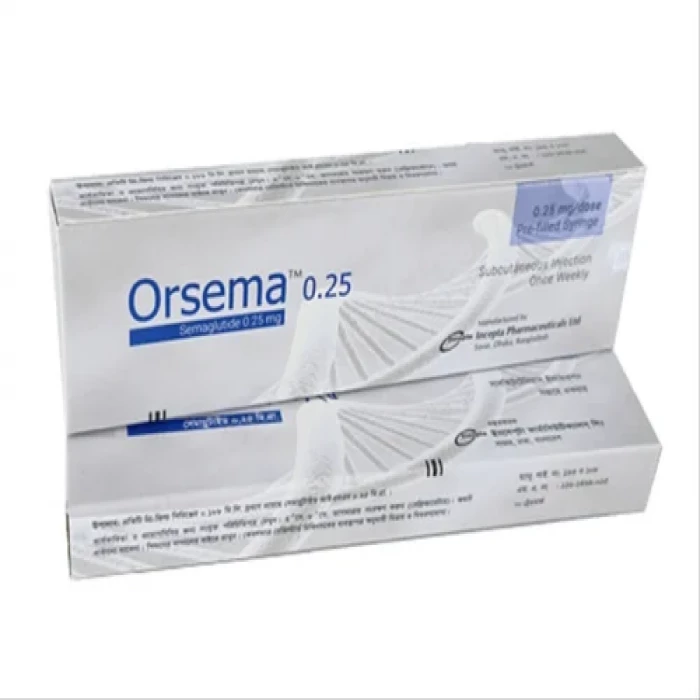
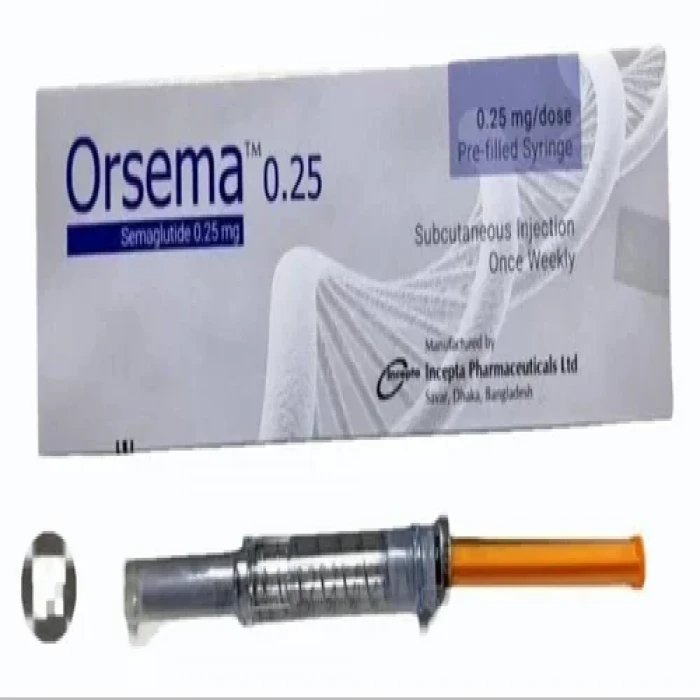
Orsema 0.25mg Subcutaneous Injection
Semaglutide is used in the treatment of type 2 diabetes mellitus.
Discount
Price: ৳ 333
MRP:
৳
350
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Orsema 0.25mg is a glucagon-like peptide 1 (GLP-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus and to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease. Additionally, it is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of 30 kg/m² or greater (Obesity) or 27 kg/m² or greater (Overweight) in the presence of at least one weight-related comorbid condition (e.g., hypertension, type 2 diabetes mellitus, or dyslipidemia).
Safety Advices

Alcohol
UNSAFE
It is not known whether it is safe to consume alcohol with Orsema 0.25mg Subcutaneous Injection. Please consult your doctor.

Pregnancy
CONSULT YOUR DOCTOR
Orsema 0.25mg Subcutaneous Injection may be unsafe to use during pregnancy. Although there are limited studies in humans, animal studies have shown harmful effects on the developing baby. Your doctor will weigh the benefits and any potential risks before prescribing it to you. Please consult your doctor.

Breastfeeding
CONSULT YOUR DOCTOR
Information regarding the use of Orsema 0.25mg Subcutaneous Injectionduring breastfeeding is not available. Please consult your doctor.

Driving
CAUTION
It is not known whether Orsema 0.25mg Subcutaneous Injection alters the ability to drive. Do not drive if you experience any symptoms that affect your ability to concentrate and react.

Kidney
SAFE IF PRESCRIBED
Orsema 0.25mg Subcutaneous Injection is safe to use in patients with kidney disease. No dose adjustment of Orsema 0.25mg Subcutaneous Injection is recommended.

Liver
SAFE IF PRESCRIBED
Orsema 0.25mg Subcutaneous Injection is safe to use in patients with liver disease. No dose adjustment of Orsema 0.25mg Subcutaneous Injection is recommended.
✔️ Uses of Orsema 0.25mg Subcutaneous Injection
Type II Diabetes mellitus.
✔️ Side Effects of Orsema 0.25mg Subcutaneous Injection
It is not known if Orsema 0.25 mg will cause thyroid tumors or medullary thyroid carcinoma (MTC) in people. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath.
✔️ Pediatric Uses:
Safety and efficacy not established in pediatric patients.
✔️ Geriatric Use:
No overall differences in safety or efficacy were detected between geriatric patients and younger patients.
✔️ Renal Impairment:
No dose adjustment is recommended.
✔️ Hepatic Impairment:
No dose adjustment is recommended.
✔️ Pharmacology
Orsema 0.25 mg is a GLP-1 analogue with 94% sequence homology to human GLP-1. It acts as a GLP-1 receptor agonist, stimulating insulin secretion, lowering glucagon secretion, and delaying gastric emptying in a glucose-dependent manner.
✔️ Dosage & Administration of Orsema 0.25mg Subcutaneous Injection
Subcutaneous injection:
- The starting dose: 0.25 mg Orsema 0.25 mg once weekly for 4 weeks subcutaneously.
- Dose Escalation:
- Week 1 through 4: 0.25 mg
- Week 5 through 8: 0.5 mg
- Week 9 through 12: 1 mg
- Week 13 through 16: 1.7 mg
- Maintenance dose: Week 17 and onwards: 2.4 mg
In the event of an overdose, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms.
✔️ Interaction
Initiating Orsema 0.25 mg concomitant with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia. Caution should be exercised when oral medications are concomitantly administered with Orsema 0.25 mg.
✔️ Contraindications
Orsema 0.25 mg is contraindicated in patients with personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) and hypersensitivity to Orsema 0.25 mg.
✔️ Pregnancy & Lactation
Limited data are available. Use during pregnancy or breastfeeding only if the potential benefit justifies the potential risk.
✔️ Precautions & Warnings
- Risk of Thyroid C-Cell Tumors.
- Pancreatitis.
- Diabetic Retinopathy Complications.
- Never Share Orsema 0.25 mg Pen Between Patients.
- Hypoglycemia with Concomitant Use of Insulin.
- Acute Kidney Injury.
- Hypersensitivity.
✔️ Storage Conditions
Store your Orsema 0.25mg Subcutaneous Injection in the refrigerator at 2°C to 8°C (36°F to 46°F).
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.