✔ 100% Authentic Product
👁️ Currently Viewing 975
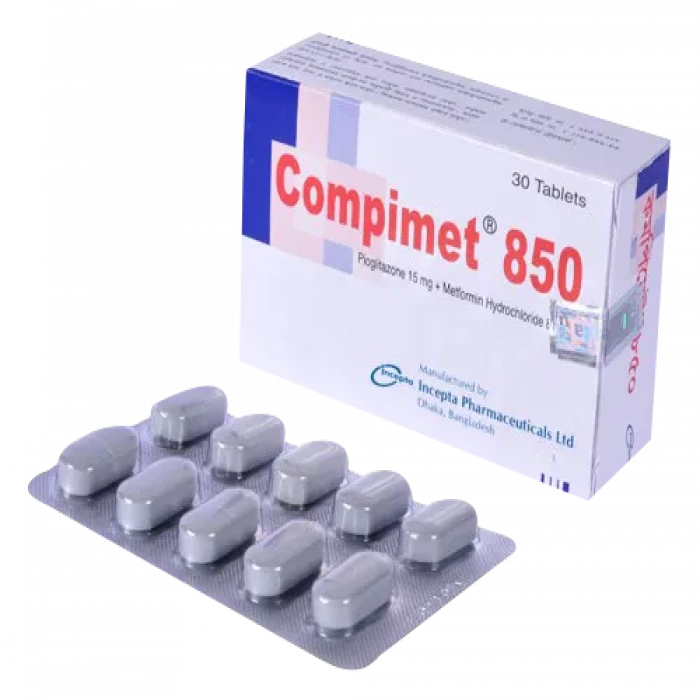
Compimet 850mg (30pcs Box)
Generic Name: Pioglitazone 15mg + Metformin 850mg,
Manufacturer: Incepta Pharmaceuticals Ltd.
Discount
Price: ৳ 310
MRP:
৳
330
6%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Indications
The combination of Pioglitazone and Metformin is used to improve glycemic control in patients with type 2 diabetes who are already taking a combination of Pioglitazone and Metformin or whose diabetes is not controlled well enough with Metformin alone, or for those who have responded well to Pioglitazone but need more glycemic control.
Pharmacology
The mechanism of action of pioglitazone is dependent on the presence of insulin. Insulin resistance in the peripheral and the liver is reduced by pioglitazone, resulting in greater insulin-dependent glucose clearance and lower hepatic glucose production. Pioglitazone is a highly specific and strong agonist for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) (PPARg). The transcription of a variety of insulin-responsive genes involved in glucose and lipid metabolism is modulated when PPARg nuclear receptors are activated.
In patients with type 2 diabetes, metformin hydrochloride improves glucose tolerance by reducing both basal and postprandial plasma glucose levels.
Dosage & Administration
General: The use of antihyperglycemic therapy in the management of type 2 diabetes should be individualized on the basis of effectiveness and tolerability while not exceeding the maximum recommended daily dose of Pioglitazone 45 mg and Metformin 2550 mg.
Dosage Recommendations: Selecting the starting dose of Pioglitazone & Metformin should be based on the patient's current regimen of Pioglitazone and/or Metformin. Pioglitazone & Metformin should be given in divided daily doses with meals to reduce the gastrointestinal side effects associated with Metformin.
Starting dose for patients inadequately controlled on Metformin monotherapy Based on the usual starting dose of Pioglitazone (15-30 mg daily), Pioglitazone & Metformin may be initiated at either the 15 mg/500 mg or 15 mg/850 mg tablet strength once or twice daily, and gradually titrated after assessing the adequacy of therapeutic response. Starting dose for patients who initially responded to Pioglitazone monotherapy and require additional glycemic control
Based on the usual starting doses of Metformin (500 mg twice daily or 850 mg daily), Pioglitazone & Metformin may be initiated at either the 15 mg/500 mg twice daily or 15 mg/850 mg tablet strength once daily, and gradually titrated after assessing the adequacy of therapeutic response.
Starting dose for patients switching from combination therapy of Pioglitazone plus Metformin as separate tablets Pioglitazone & Metformin may be initiated with either the 15 mg/500 mg or 15 mg/850 mg tablet strengths based on the dose of Pioglitazone and Metformin already being taken.
Interaction
Absorption of vitamin B12 may be hampered. Strong CYP2C8 inhibitors result in higher plasma levels (e.g. gemfibrozil). CYP2C8 inducers resulted in lower plasma levels (e.g. rifampicin). Cationic medicines cause a reduction in metabolism, which is then removed through renal tubular secretion.
Contraindications
Renal illness or renal failure (e.g., as evidenced by serum creatinine levels >1.5 mg/dl in males, >1.4 mg/dl in females, or inadequate creatinine clearance) is contraindicated in patients with cardiovascular collapse (shock), acute myocardial infarction, or septicemia.
Hypersensitivity to Pioglitazone, Metformin, or any other ingredient in this combination.
Diabetic ketoacidosis with or without coma, whether acute or chronic metabolic acidosis.
Side Effects
This mixed preparation is often well tolerated. Upper respiratory tract infection, diarrhea, peripheral edema, and headache are the most prevalent adverse effects, correspondingly. In terms of severity, these are minor.
Pregnancy & Lactation
. Pregnancy: There have been no adequate and well-controlled trials of this combination or its separate components in pregnant women. As a result, it should only be used when the potential benefit outweighs the danger to the fetus.
Pioglitazone and/or Metformin are not known to be secreted in human milk by nursing mothers. This combination should not be given to a lactating mother because many medications are excreted in human milk.
Precautions & Warnings
Only in the presence of insulin can pioglitazone have an antihyperglycemic action. As a result, Pioglitazone should not be used to treat diabetic ketoacidosis in patients with type 1 diabetes. When used with insulin, hepatic insufficiency, or heart disease, pioglitazone should be administered with caution.
Metformin is known to be eliminated in large amounts through the kidneys, and the danger of Metformin buildup and lactic acidosis increases as renal function declines. Patients whose serum creatinine levels are higher than the upper limit of normal for their age should not take this medication.
Storage Conditions
Keep the temperature below 30°C and away from light and moisture. Keep out of children's reach.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.