✔ 100% Authentic Product
👁️ Currently Viewing 2169
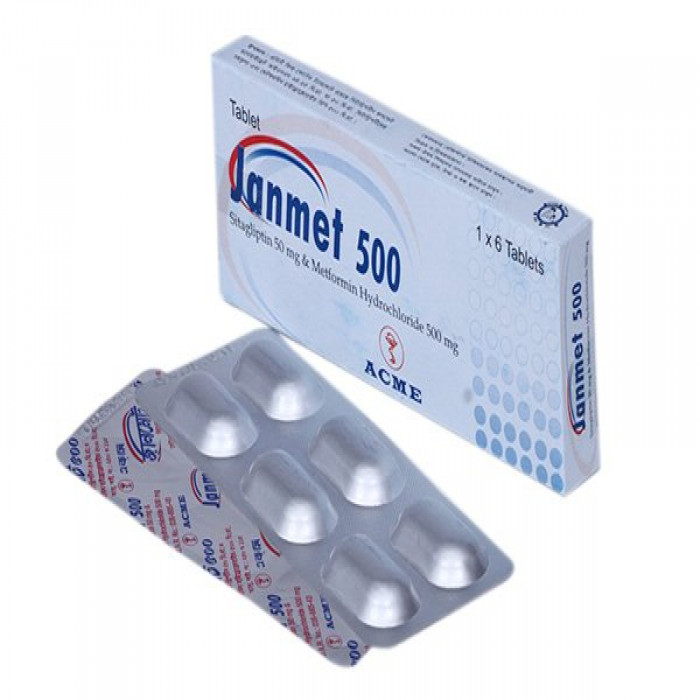
Janmet 500mg 10pcs
Tablet Manufacturer/Distributor: Acme Laboratories Ltd. Generic Name: Metformin Hydrochloride 500 mg + Sitagliptin 50 mg Tablet
Discount
Price: ৳ 169
MRP:
৳
180
6%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Indications
Janmet is an oral anti-diabetic drug that helps control blood sugar levels. Janmet contains a combination of Metformin and Sitagliptin. Metformin and Sitagliptin are oral diabetes medicines that help control blood sugar levels. Janmet is used along with diet and exercise to lower blood sugar in adults with type 2 diabetes (NIDDM). Composition Janmet 500 : Each film-coated tablet contains Sitagliptin Phosphate Monohydrate INN 64.25 mg equivalent to 50 mg of Sitagliptin and Metformin Hydrochloride BP 500 mg. Janmet 1000 : Each film-coated tablet contains Sitagliptin Phosphate Monohydrate INN 64.25 mg equivalent to 50 mg of Sitagliptin and Metformin Hydrochloride BP 1000 mg.
Pharmacology
This tablet combines two antihyperglycemic agents with complementary mechanisms of action to help patients with type 2 diabetes improve their glycemic control. Metformin HCl, a member of the biguanide class, and Sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor. Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that is thought to work in type 2 diabetes patients by reducing the inactivation of incretin hormones. The intestine releases incretin hormones throughout the day, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), and levels rise in response to a meal.
DPP-4, an enzyme, rapidly inactivates these hormones. The incretins are part of an endogenous system that regulates glucose homeostasis in the body. GLP-1 and GIP stimulate insulin production and release from pancreatic beta cells via intracellular signaling pathways including cyclic AMP when blood glucose levels are normal or increased. GLP-1 also inhibits the secretion of glucagon by pancreatic alpha cells, resulting in lower hepatic glucose synthesis. Sitagliptin enhances insulin release and reduces glucagon levels in the circulation in a glucose-dependent manner by boosting and prolonging active incretin levels. Metformin HCl has a different pharmacologic mode of action than other oral antihyperglycemic drugs. Metformin HCl reduces hepatic glucose synthesis, decreases glucose absorption in the intestine, and improves glucose uptake and utilization in the peripheral tissues.
Dosage and Administration
Janmet should generally be given twice daily with meals, with gradual dose escalation, to reduce the gastrointestinal (GI) side effects due to Metformin. The recommended starting dose in patients not currently treated with Metformin is 50 mg Sitagliptin/500 mg Metformin hydrochloride twice daily. The starting dose in patients already treated with Metformin should provide Sitagliptin dosed as 50 mg twice daily (100 mg total daily dose) and the dose of Metformin already being taken. For patients taking Metformin 850 mg twice daily, the recommended starting dose of Janmet is 50 mg Sitagliptin/1000 mg Metformin twice daily. Patients treated with an insulin secretagogue or insulin Co-administration of Janmet with an insulin secretagogue (e.g., sulfonylurea) or insulin may require lower doses of the insulin secretagogue or insulin to reduce the risk of hypoglycemia. Use in Specific Populations Pediatric population- There is no data on the use of Sitagliptin in patients younger than 18 years of age and therefore not recommended. Geriatric Use- Janmet should be used with caution as age increases. Care should be taken in dose selection and should be based on careful and regular monitoring of renal function. Renal Insufficiency- A dosage adjustment is recommended in patients with moderate or severe renal insufficiency and in patients with ESRD requiring hemodialysis or peritoneal dialysis. Impaired Hepatic Function- Janmet should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Use in Pancreatitis: Janmet has not been studied in patients with a history of pancreatitis. It is unknown whether patients with a history of pancreatitis are at increased risk of developing pancreatitis while taking Janmet. OR AS DIRECTED BY THE PHYSICIAN.
Interaction
Cationic agents that are eliminated by renal tubular secretion (e.g. cimetidine) may interact with Metformin by competing for common renal tubular transport systems. So, close monitoring of glycaemic control, dose adjustment within the recommended posology and changes in diabetic treatment should be considered when cationic agents that are eliminated by renal tubular secretion are co-administered. Janmet may also have some drug interactions with Digoxin, Glyburide, Furosemide and Nifedipine and need close monitoring when treating NIDDM patient. The Use of Metformin with Other Drugs Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. When such drugs are administered to a patient receiving Janmet the patient should be closely observed to maintain adequate glycemic control. Supply Janmet 500 : Each box contains 1x6 tablets in Alu-Alu blister strip. Janmet 1000 : Each box contains 1x6 tablets in Alu-Alu blister strip. Keep all medicines out of reach of the children.
Contraindication
Janmet is contraindicated in patients with hypersensitivity to the active substances or to any of the excipients, diabetic ketoacidosis, diabetic pre-coma, moderate and severe renal impairment [serum creatinine levels ³ 1.5 mg/dL (males), ³ 1.4 mg/dL (Females)]. Precaution Janmet may cause lactic acidosis, low blood sugar, and decreased vitamin B12 levels. Janmet warnings and precautions also extend to those who are allergic to any components of the drug, have metabolic or diabetic ketoacidosis, have kidney disease, or are about to have an x-ray procedure with contrast dye.
Side Effects
Most common side effects of Janmet are diarrhea, gas, headache, indigestion, nausea, sore throat, stomach upset, stuffy or runny nose, vomiting, and weakness. Severe allergic reactions (rash; hives; itching; difficulty swallowing or breathing; tightness in the chest; swelling of the mouth, face, lips, throat, or tongue; unusual hoarseness); chest pain or discomfort; decreased urination; dizziness or light-headedness; fast or difficult breathing; feeling of being unusually cold; general feeling of being unwell; muscle pain or weakness; red, blistered, swollen, or peeling skin; slow or irregular heartbeat; symptoms of pancreas inflammation (eg, severe stomach or back pain with or without nausea or vomiting); unusual drowsiness; unusual or persistent stomach pain or discomfort; unusual tiredness or weakness.
Pregnancy and Lactation
Pregnancy Category : B. Animal studies have failed to reveal evidence of fetal harm. There are no controlled data in human pregnancy. Sitagliptin-Metformin is only recommended for use during pregnancy when benefit outweighs risk. Lactation There are no data on the excretion of Sitagliptin-Metformin into human milk. It is recommended that caution should be used when administering Sitagliptin-Metformin to nursing women.
Precautions & Warnings
Lactic Acidosis is a condition in which the body produces too much lactate.
Metformin buildup can lead to lactic acidosis. The risk increases with conditions such as sepsis, dehydration, excess alcohol intake, hepatic insufficiency, renal impairment, and acute congestive heart failure. Malaise, myalgias, respiratory discomfort, increased somnolence, and nonspecific stomach distress are some of the symptoms. Low pH, increased anion gap, and high blood lactate are examples of laboratory abnormalities.
If acidosis is suspected, stop taking this tablet and admit the patient to the hospital right away. Patients with hypothyroidism should have their thyroid-stimulating hormone (TSH) levels checked regularly.
Long-term metformin medication has been linked to a drop in vitamin B12 serum levels, which can lead to peripheral neuropathy. Monitoring of the vitamin B12 level is recommended.
Storage conditions
Store in a cool and dry place protected from light.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.