
✔ 100% Authentic Product
👁️ Currently Viewing 3053
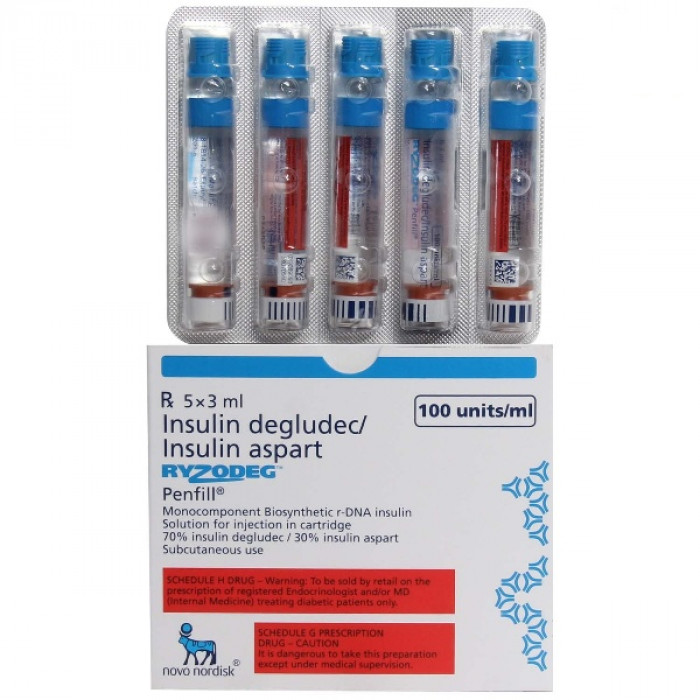
Ryzodeg 100IU Penfill Cartridge
- Ryzodeg Penfill is a combination of two insulins used to treat type 1 and type 2 diabetes in adults and children.
- It helps control blood sugar levels by providing a steady level of insulin in the body.
- Use as instructed by your healthcare provider, along with a healthy diet and exercise regimen.
🚚 তাপমাত্রা নিয়ন্ত্রিত ডেলিভারি: এই পণ্যটি গুণগত মান বজায় রাখতে শুধু মাত্র ঢাকা শহরের ভিতরে আইস ব্যাগ এর মাধ্যমে ডেলিভারি করা হয়।
Discount
Price: ৳ 1,285
MRP:
৳
1325
3%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Ryzodeg 100IU Penfill Cartridge is a combination of Insulin Aspart and Insulin Degludec, which are classified as Antidiabetic agents. It is prescribed to enhance glycemic control in patients diagnosed with diabetes mellitus, including both Type I and Type II diabetes.
Diabetes mellitus is a chronic condition characterized by elevated levels of glucose in the bloodstream. This can occur due to insufficient production of insulin, a hormone responsible for metabolizing glucose, by the pancreas, or due to reduced sensitivity of body cells to the actions of insulin.
Ryzodeg 100IU Penfill Cartridge is suitable for use in both adults and children aged 6 years and above. It is administered via subcutaneous injection, typically in areas such as the thigh, upper arm, or abdomen. It's important to avoid sharing needles to prevent the risk of infections. Additionally, adopting a healthy lifestyle, including abstaining from smoking and alcohol consumption, and following a balanced diet with regular physical activity, is advised in conjunction with this treatment as recommended by your doctor.
Before initiating therapy with Ryzodeg 100IU Penfill Cartridge, it's crucial to discuss with your doctor if you have any underlying liver, kidney, or heart conditions. Inform your doctor about any hormonal disorders you may have, recent changes to your diet, or if you engage in intense exercise routines as a precautionary measure.
Do not use this medication if you are experiencing hypoglycemia (low blood sugar levels). If you are pregnant or breastfeeding, consult your doctor before using Ryzodeg 100IU Penfill Cartridge.
Safety Advices

Alcohol
UNSAFE
It's unsafe to consume alcohol while using Ryzodeg Penfill.

Pregnancy
CONSULT YOUR DOCTOR
Consult your doctor before using Ryzodeg Penfill during pregnancy due to potential risks to the developing baby.

Breastfeeding
CONSULT YOUR DOCTOR
Ryzodeg Penfill is safe to use during breastfeeding if prescribed by a doctor.

Driving
CAUTION
Be cautious while driving if your blood sugar levels are too low or too high, as it may affect your ability to drive.

Kidney
CAUTION
Use Ryzodeg Penfill with caution in patients with kidney disease. Dose adjustment may be necessary, and regular monitoring of blood glucose levels is recommended.

Liver
CAUTION
Exercise caution when using Ryzodeg Penfill in patients with liver disease. Dose adjustment may be required, and frequent monitoring of blood glucose levels is advised.
✔️ Uses of Ryzodeg 100IU Penfill Cartridge
Ryzodeg Penfill is used to treat diabetes mellitus (type 1 & type 2).
✔️ How does Ryzodeg 100IU Penfill Cartridge work?
Ryzodeg Penfill combines two insulins: insulin degludec and insulin aspart, which work together to control blood sugar levels by facilitating glucose uptake and suppressing liver sugar production.
✔️ Side Effects of Ryzodeg 100IU Penfill Cartridge
- Common side effects include nasopharyngitis, severe hypoglycemia, headache, upper respiratory tract infection, influenza, peripheral edema, and injection site reactions.
- Allergic reactions and lipodystrophy may occur rarely.
✔️ Quick Suggestions:
- Ryzodeg SC Injection is indicated to improve glycemic control in patients aged 1 year and older with diabetes mellitus.
- It can be used in patients with renal and hepatic impairment, with adjustments in insulin dose and intensified glucose monitoring.
- Patients should be instructed to check the insulin label before each injection to avoid mix-ups.
- Glucose monitoring and insulin dose adjustments may be needed in older patients and those with renal or hepatic impairment.
✔️ Indications:
Ryzodeg SC Injection is a combination of Insulin Degludec and Insulin Aspart, both human insulin analogs.
✔️ Pharmacology
Insulin Degludec and Insulin Aspart bind to the human insulin receptor, resulting in the same pharmacological effects as human insulin. They facilitate the uptake of glucose into muscle and fat cells while inhibiting glucose output from the liver.
✔️ Dosage & Administration:
Ryzodeg SC Injection is a soluble insulin product consisting of basal insulin degludec and rapid-acting prandial insulin aspart. The dosage is individualized based on the patient's needs, primarily adjusted according to fasting plasma glucose measurements.
The injection can be administered once- or twice daily with the main meal(s). In type 2 diabetes mellitus, it can be used alone, in combination with oral anti-diabetic medications, or with bolus insulin. In type 1 diabetes mellitus, it is combined with short-/rapid-acting insulin at other meals.
- Your healthcare provider will guide you on how to use Ryzodeg Penfill properly.
- It's typically taken 15 minutes before a meal or within 20 minutes after starting a meal.
- Injection sites should be rotated to prevent lumps from developing.
- Monitor blood sugar levels regularly and adjust the dose as needed.
✔️ Interaction
Before using Ryzodeg 100IU Penfill Cartridge, inform your doctor if you are taking any of the following medications:
- Other antidiabetic agents: (e.g., tolbutamide, glibenclamide, glipizide)
- Biguanides: (e.g., metformin, phenformin)
- Thiazolidinediones: (e.g., pioglitazone)
- DPP-4 inhibitors: (e.g., sitagliptin, vildagliptin)
- SGLT-2 inhibitors: (e.g., remogliflozin)
- Antidepressants: (e.g., rasagiline, selegiline, phenelzine)
- Heart medicines: (e.g., propranolol, atenolol, nebivolol)
- ACE inhibitors: (e.g., captopril, enalapril, lisinopril)
- Salicylates: (e.g., aspirin)
- Anabolic steroids: (e.g., testosterone, nandrolone, oxandrolone)
- Sulfonamide antibiotics: (e.g., sulfasalazine, sulfadiazine, sulfamethoxazole)
- Oral contraceptives: (e.g., mifepristone)
- Thiazide diuretics: (e.g., chlorothiazide, hydrochlorothiazide)
- Glucocorticoids: (e.g., cortisone, betamethasone, dexamethasone)
- Thyroid hormone: (e.g., thyroxine, triiodothyronine)
- Sympathomimetic agents: (e.g., ephedrine, adrenaline, salbutamol)
- Growth hormone
- Danazol
- Octreotide
- Lanreotide
Interaction with Food or Drink: Ryzodeg 100IU Penfill Cartridge should be administered 30 minutes before a meal.
✔️ Contraindications
Hypersensitivity to the active substances or any excipients is a contraindication for its use.
✔️ Pregnancy & Lactation
It falls under Pregnancy Category C, meaning animal studies have shown adverse effects on the fetus. There are no adequate and well-controlled studies in humans. It may be used in pregnant women if the potential benefits outweigh the potential risks.
✔️ Precautions & Warnings
- Be cautious of hypoglycemia, especially during illness or changes in physical activity.
- Hyperglycemia may occur if the insulin dose is too low.
- Patients with eye disorders should be aware of potential changes in diabetic retinopathy.
- Avoid accidental mix-ups with other insulin products.
- Older patients (>65 years old) can use Ryzodeg Penfill with intensified glucose monitoring.
- Safety and efficacy in children and adolescents have not been established.
✔️ Storage Conditions
- Keep out of reach of children
- Store the unopened pack in the refrigerator at 2-8°C. Do not freeze
- During use or when carried as a spare, keep it at room temperature
- Always place the vial/cartridge in the outer carton to protect it from light
- Do not use the medicine after 28 days of opening
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.