✔ 100% Authentic Product
👁️ Currently Viewing 3417
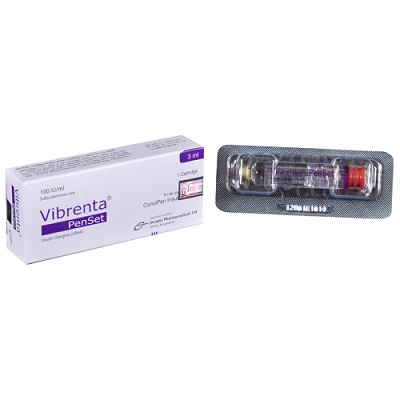
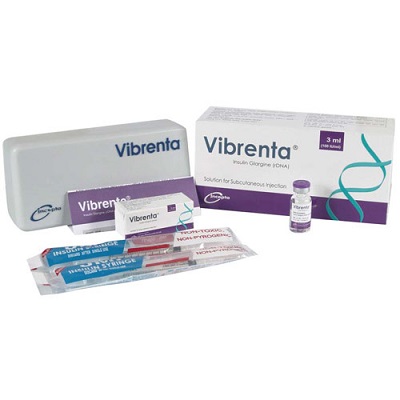
Vibrenta Vial (100IU/ml)
Generic Name: Insulin Glargine (rDNA) 100 IU/ml.
Manufacturer: Incepta Pharmaceuticals Limited
Discount
Price: ৳ 570
MRP:
৳
600
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Indications
Insulin Glargine is indicated to improve glycemic control in adults and children with type 1 diabetes mellitus and in adults with type 2 diabetes mellitus.
Therapeutic Class
Long Acting Insulin
Pharmacology
Insulin Glargine is a sterile solution of insulin glargine for use as a subcutaneous injection. Insulin glargine is a recombinant human insulin analogue that is a long-acting (up to 24-hour duration of action), parenteral blood glucose lowering agent. Insulin Glargine is produced by recombinant DNA technology. Primary function of insulin glargine is regulation of glucose metabolism. Insulin and its analogues lower blood glucose by stimulation peripheral glucose uptake, primarily by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis and proteolysis and enhances protein synthesis.
Dosage
Insulin Glargine exhibits a relatively constant glucose-lowering profile over 24 hours that permits once-daily dosing. Potency of insulin glargine is approximately the same as human insulin.
Insulin Glargine is recommended for once daily subcutaneous administration & may be administered at any time during the day. However, once started should be administered at the same time every day. The dose of Insulin Glargine must be individualized based on clinical response. Blood glucose monitoring is essential in all patients with diabetes. In patients with type 1 diabetes, Insulin Glargine must be used in regimens with short-acting insulin. Insulin Glargine is not recommended for intravenous administration. Intravenous administration of the usual subcutaneous dose could result in severe hypoglycemia.
Initiation of Insulin Glargine therapy:
- The recommended starting dose of Insulin Glargine in patients with type 1 diabetes should be approximately one-third of the total daily insulin requirements. Short-acting, premeal insulin should be used to satisfy the remainder of the daily insulin requirements.
- The recommended starting dose of Insulin Glargine in patients with type 2 diabetes who are not currently treated with insulin is 10 units (or 0.2 Units/kg) once daily, which should subsequently be adjusted to the patient’s needs.
Converting to Insulin Glargine from other insulin therapies: If changing from a treatment regimen with an intermediate-or long-acting insulin to a regimen with Insulin Glargine , the amount and timing of shorter-acting insulins and doses of any oral anti-diabetic drugs may need to be adjusted.
- If transferring patients from once-daily NPH insulin to once-daily Insulin Glargine , the recommended initial Insulin Glargine dose is the same as the dose of NPH that is being discontinued.
- If transferring patients from twice-daily NPH insulin to once-daily Insulin Glargine , the recommended initial Insulin Glargine dose is 80% of the total NPH dose that is being discontinued.
Administration
Insulin Glargine should be injected subcutaneously once daily at any time of day, but at the same time everyday.
Cartridge:
- Insert the Insulin Glargine cartridge into the pen correctly and equip the needle. Gently turn the pen upside down for 8-10 times until the insulin in the cartridge becomes uniformly mixed.
- Adjust the dosage button to get correct dose. After removal of the needle cap and discharge air bubbles in the cartridge, it is ready to be injected. In order to avoid cross contamination, do not let the needle touch anything during the process of preparation.
Vial:
- Firstly, clean your hands. Shake or rotate the vial gently to mix the solution uniformly and check if the insulin has the normal appearance.
- If using a new Insulin Glargine bottle then flip off the plastic protective cap and wipe the rubber stopper with an alcohol swab.
- Draw air into your syringe equal to the amount of Insulin Glargine needed. Puncture the needle into the vial and inject the air.
- Turn the bottle and syringe upside down. Withdraw correct dose of Insulin Glargine into the syringe. Before pulling out the needle, check if there are any bubbles remain in the syringe.
- If so, put the syringe upright and tap the syringe to discharge the air bubbles.
Injection Site: Choose the area where skin is less tight, such as the upper arm, thigh, buttock and abdomen, etc. To avoid tissue damage, choose a site for each injection that is at least 1 cm from the previous injection site.
Injection Method: Cleanse the skin with alcohol where the injection is to be made. Put the needle in such a position as to form 45° angle with the skin. Puncture the needle into skin and inject insulin. Then pull the needle out and apply gentle pressure over the injected site for several seconds. Do not rub the injection site.
Interaction
A number of drugs affect glucose metabolism and may require dose adjustment.
The following substances may reduce the Insulin as well as Insulin glargine requirements: Oral anti-diabetic products, angiotensin converting enzyme (ACE) inhibitors, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, propoxyphene, pentoxifylline, salicylates and sulfonamide antibiotics.
The following substances may increase the Insulin as well as Insulin glargine requirements: Thiazides, glucocorticoids, thyroid hormones, beta-sympathomimetics, growth hormone and danazol. Beta-blockers, clonidine, lithium salts, and alcohol may either potentiate or weaken the blood-glucose-lowering effect of insulin.
Contraindications
Insulin glargine is contraindicated in patients with hypersensitivity to insulin glargine or any of its excipients.
Side Effects
Side effects of Insulin glargine are hypoglycemia, allergic reactions, injection site reaction, lipodystrophy, pruritus, and rash.
Pregnancy & Lactation
Pregnancy category C. Insulin glargine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Lactation: It is unknown whether insulin glargine is excreted in human milk. Because many drugs, including human insulin, are excreted in human milk, caution should be exercised when Insulin glargine is administered to a nursing woman. Lactating women may require adjustments in insulin dose & diet.
Precautions & Warnings
Dose adjustment and monitoring: Blood glucose should be monitored in all patients treated with insulin. Insulin regimens should be modified cautiously and only under medical supervision.
Administration: Insulin glargine must not be diluted or mixed with any other insulin or solution. It should not be administered subcutaneously via an insulin pump or intravenously because severe hypoglycemia can occur.
Renal or hepatic impairment: Reduction in the Insulin glarginedose may require in these cases.
Use in Special Populations
Use in Renal/ Hepatic impairment: Reduction in the insulin glargine doses may be required in these cases.
Overdose Effects
Insulin glargine overdose may result in hypoglycemia. Mild episodes of hypoglycemia can usually be treated with oral carbohydrates. Severe hypoglycemia may be treated with parenteral glucose or injections of glucagon. Adjustments in drug dosage, meal patterns, or exercise may be needed.
Storage Conditions
Store at 2° C to 8° C in a refrigerator. Do not freeze. In case of insulin for recent use need not to be refrigerated, try to keep it in a cool place and keep away from heat and light. The insulin in use can be kept under the room temperature for a month.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.