✔ 100% Authentic Product
👁️ Currently Viewing 1872
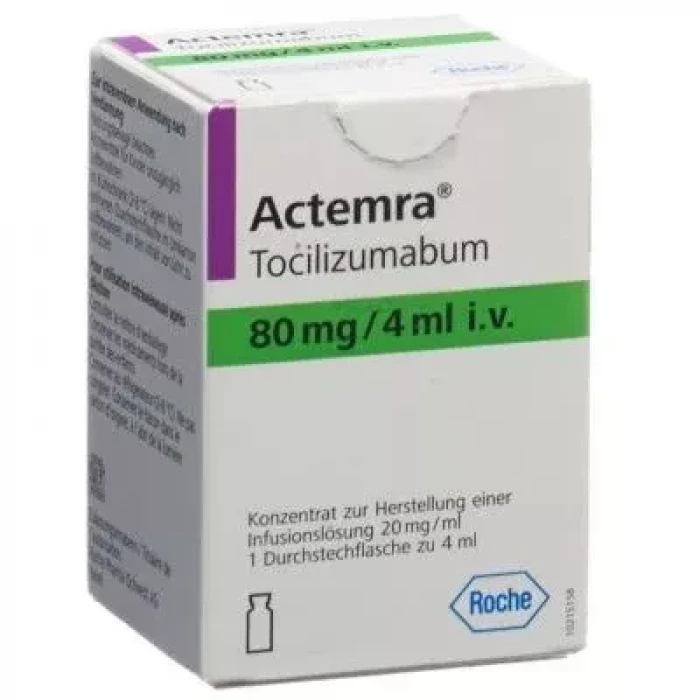
Actemra 80mg/4ml IV Infusion
- Treatment of Ankylosing spondylitis
- Treatment of Rheumatoid arthritis
- Treatment of Psoriasis
- Treatment of Ulcerative colitis
- Treatment of Crohn’s disease
Discount
Price: ৳ 9,100
MRP:
৳
10301
11.66%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
- Rheumatoid Arthritis: An autoimmune disease where the body's immune system mistakenly attacks its tissues, leading to joint pain and damage. Common symptoms include pain, swelling, stiffness, joint deformities, and a loss of joint function.
- Ankylosing Spondylitis: A chronic condition primarily affecting the spine, causing pain and stiffness that usually begins in the lower back and can extend to the neck, joints, and other parts of the body. Symptoms include reduced flexibility, which can result in a hunched-forward posture and persistent pain in the back and joints.
- COVID-19: A respiratory illness caused by the SARS-CoV-2 virus, transmitted through droplets from coughs, sneezes, or exhalation of an infected person. Common symptoms include fever, dry cough, fatigue, body aches, sore throat, and loss of taste and smell. Severe cases can lead to complications like pneumonia, respiratory failure, heart and liver problems, septic shock, and death, often marked by low oxygen levels, shortness of breath, chest pain, high fever, and extreme weakness.
Safety Advices

Alcohol
UNSAFE
You are recommended to avoid alcohol consumption if you are being treated with Actemra 80mg/4ml IV Infusion. Alcohol intake, along with Actemra 80mg/4ml IV Infusion, may cause increased dizziness and risk of stomach bleeding/ulcers.

Pregnancy
CONSULT YOUR DOCTOR
Actemra 80mg/4ml IV InfusionN is not recommended for use in pregnant women unless clearly necessary. Women of childbearing potential must use effective contraception during and up to 3 months after> management. Contact your doctor before receiving Actemra 80mg/4ml IV Infusion.

Breastfeeding
CONSULT YOUR DOCTOR
Actemra 80mg/4ml IV Infusion is not recommended for use in breastfeeding mothers as it is not known if it is excreted in the breast milk. Leave a gap of at least 3 months after your last management before starting breastfeeding. Consult your doctor before receiving Actemra 80mg/4ml IV Infusion.

Driving
CAUTION
Do not drive or operate any machines if you feel dizzy after receiving Actemra 80mg/4ml IV Infusion.

Kidney
CONSULT YOUR DOCTOR
Actemra 80mg/4ml IV Infusion should be used with caution in patients with mild to moderate kidney disease with no dose adjustment. There is no information available on the use of this medicine in severe kidney disease. Consult your doctor before receiving Actemra 80mg/4ml IV Infusion.

Liver
CONSULT YOUR DOCTOR
Actemra 80mg/4ml IV Infusion is not recommended in patients with active liver disease or liver impairment. Consult your doctor before receiving Actemra 80mg/4ml IV Infusion.
✔️ Uses of Actemra 80mg/4ml IV Infusion
Treatment of Rheumatoid arthritis, Ankylosing spondylitis, and giant cell arteritis.
✔️ How does Actemra 80mg/4ml IV Infusion work?
Actemra 80mg/4ml IV Infusion works by blocking the activity of a specific protein (cytokine) called interleukin 6, which is involved in inflammatory processes of the body, and blocking it can reduce the pain and swelling in your joints.
✔️ Side Effects of Actemra 80mg/4ml IV Infusion
- Runny nose
- Sinus pain
- Sore throat
- Increased blood pressure
- Pain and swelling at the injection site
- Headache
✔️ Quick Suggestions:
- Actemra 80mg/4ml IV Infusion is given as a drip (intravenous infusion) or as an injection directly into a vein.
- During the treatment, you may be asked for regular blood tests to check blood counts, cholesterol levels,s, and liver function.
- It might make you feel dizzy. If this happens, avoid driving or operating on machinery.
- It makes it hard to fight with an infection. Inform your doctor if you notice fever, cough, or stomach pain.
- Inform your doctor if you are pregnant, planning to become pregnant, or are breastfeeding.
- Do not stop taking medicine without talking to your doctor first.
✔️ Indication of Actemra 80mg/4ml IV Infusion
Actemra 80mg/4ml IV Infusion contains Tocilizumab which belongs to the group of medicines called interleukin-6 (IL-6) receptor inhibitors. It is used to manage adults with moderate to severely active rheumatoid arthritis (an inflammatory disease affecting the joints).
✔️ Pharmacology
Tocilizumab is a humanized monoclonal antibody that targets and inhibits the interleukin-6 (IL-6) receptor, which is part of the immunoglobulin IgG1K subclass. By binding to both soluble and membrane-bound IL-6 receptors, Tocilizumab effectively blocks IL-6-mediated signaling. IL-6 is a key pro-inflammatory cytokine produced by various cells, including T-cells, B-cells, monocytes, and fibroblasts. It plays a significant role in immune system functions like T-cell activation, antibody production, liver protein synthesis, and blood cell formation. Additionally, IL-6 is involved in local inflammatory processes in the joints, contributing to conditions like rheumatoid arthritis.
✔️ Dosage & Administration of Actemra 80mg/4ml IV Infusion
Intravenous (IV) Preparation:
Preparation:
Administration:
|
Subcutaneous (SC) Preparation and Administration:
Preparation:
Administration:
|
Dosage Recommendations:
Adult Dose:
Rheumatoid Arthritis:
- IV Infusion:
- Start with 4 mg/kg every 4 weeks.
- May increase to 8 mg/kg every 4 weeks based on clinical response.
- Maximum dose: 800 mg per infusion.
- SC Injection:
- Weight < 100 kg: 162 mg SC every other week, increasing to every week based on clinical response.
- Weight > 100 kg: 162 mg SC every week.
Hepatic Impairment:
- Not recommended in patients with active hepatic disease or hepatic impairment.
Child Dose:
Systemic Juvenile Idiopathic Arthritis (SJIA):
- < 2 years: Safety and efficacy not established.
- > 2 years:
- Weight < 30 kg: 12 mg/kg IV every 2 weeks.
- Weight > 30 kg: 8 mg/kg IV every 2 weeks.
- Can be administered as monotherapy or with methotrexate.
Polyarticular Juvenile Idiopathic Arthritis (PJIA):
- < 2 years: Safety and efficacy not established.
- > 2 years:
- Weight < 30 kg: 10 mg/kg IV every 4 weeks.
- Weight > 30 kg: 8 mg/kg IV every 4 weeks.
- Can be administered as monotherapy or with methotrexate.
Renal Dose:
- Mild Renal Impairment: No dosage adjustment is required.
- Moderate-to-severe renal Impairment: Has not been studied.
✔️ Drug Interactions with Tocilizumab
Drug-Drug Interactions:
- Immunosuppressants: Tocilizumab may interact with other immunosuppressants such as certolizumab, golimumab, infliximab, adalimumab, and rituximab.
- Rheumatoid Arthritis Medications: Be cautious when combining Tocilizumab with other rheumatoid arthritis medications like Abatacept, etanercept, and Anakinra.
- Blood Thinners: It can interact with blood thinners such as warfarin, potentially affecting their efficacy.
- Cholesterol-Lowering Drugs: Tocilizumab may interact with cholesterol-lowering medications like atorvastatin, lovastatin, and simvastatin.
- Acid-Reducing Medications: Interaction with drugs used to treat acidity, such as omeprazole, may occur.
- Cough Suppressants: Tocilizumab can interact with cough suppressants like dextromethorphan.
- Birth Control Pills: Tocilizumab may reduce the effectiveness of oral contraceptives.
Drug-Food Interaction:
- Alcohol: Consuming alcohol while on Tocilizumab can increase dizziness and raise the risk of stomach ulcers and bleeding.
Drug-Disease Interactions:
- Caution Required: Use Tocilizumab cautiously if you have liver, heart, or kidney diseases, stomach or intestinal ulcers, multiple sclerosis, low blood cell count, diabetes, a weakened immune system (e.g., HIV), hepatitis B, cancer, or active infections.
✔️ Contraindications
Tocilizumab is contraindicated in patients with known hypersensitivity to Tocilizumab.
✔️ Pregnancy & Lactation
Based on animal data, may cause fetal harm. In case of COVID-19, Tocilizumab should be used during pregnancy only if the potential benefit justifies the potential risk for the mother and the fetus. Discontinue drug or nursing taking into consideration importance of drug to mother. Breastfeeding individuals with COVID-19 should follow practices according to clinical guidelines to avoid exposing the infant to COVID-19.
✔️ Drug Warnings for Tocilizumab
- Pre-Existing Conditions: Before starting Tocilizumab, inform your doctor if you have any liver, heart, or kidney diseases, stomach or intestinal ulcers (such as diverticulitis), multiple sclerosis, low blood cell count, diabetes, a weakened immune system (e.g., HIV), hepatitis B, cancer, or any active infections.
- Infection Precautions: Avoid close contact with individuals who have contagious infections like chickenpox, measles, tuberculosis, or the flu. Practice good hygiene, including thorough handwashing, to prevent the spread of infection.
- Vaccinations: Do not receive live vaccines while being treated with Tocilizumab, as this could lead to serious complications.
- Alcohol and Driving: Tocilizumab may cause dizziness or drowsiness. Avoid alcohol, as it can exacerbate these effects and increase the risk of stomach bleeding or ulcers. Only drive if you feel alert and focused.
- Pediatric Use: Tocilizumab is not recommended for children under two years of age as its safety and efficacy in this age group have not been established.
- Storage: Store Tocilizumab at temperatures between 2°C and 8°C. Do not freeze the vial.
✔️ Storage:
- Keep Actemra 80mg/4ml IV Infusion out of reach from children
- Store Actemra 80mg/4ml IV Infusion in a refrigerator (2°C - 8°C)
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.