
✔ 100% Authentic Product
👁️ Currently Viewing 1789
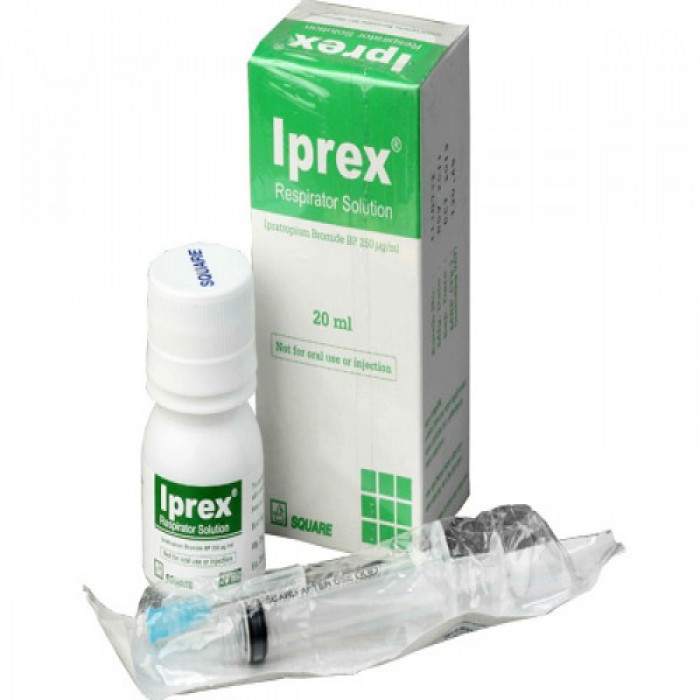
Type: Inhaler Generic Name:Ipratropium broml0e 250mcg/ml: Respirator solution for inhalation. Manufacturer/Distributor: Square
Discount
Price: ৳ 124
MRP:
৳
130.88
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Indications
Ipratropium bromide inhalation solution administered either alone or with other bronchodilators, especially beta adrenergics, is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema.
Pharmacology
Ipratropium Bromide may be a quaternary ammonium compound with anticholinergic properties. It shows up to repress vagal reflexes by offending the activity of acetylcholine (the transmitter specialist discharged from the vagus nerve). Anticholinergics avoid the increment in intracellular concentration of cyclic guanosine monophosphate (cyclic GMP) caused by interaction of acetylcholine with the muscarinic receptor on bronchial smooth muscle.
Dosage & Administration
Adults -
Iprex® respirator Solution 0.4-2 ml (100-500 mcg) of Ipratropium bromide should be diluted to a final volume of 2.0-4.0 ml with normal saline 0.9% administered four times daily. It is advisable to use the resultant solution within 24 hours from time of dilution when stored at room temperature and within 48 hours when stored in the refrigerator. The resultant solution is inhaled from a suitably driven nebulizer until aerosol generation ceases. Using a correctly matched nebulizer and driving source, this should take about 10 minutes.
Children (over 3 years) -
The same mode of administration is applicable to children.0.4-2 ml of the prepared solution administered 3 times daily.
Interaction
No information available.
Contraindications
Ipratropium bromide inhalation aerosol is contraindicated in patients with a history of hypersensitivity to soya lecithin or related food products such as soybean and peanut. Ipratropium bromide is contraindicated in known or suspected cases of hypersensitivity to ipratropium bromide, or to atropine and its derivatives. Ipratropium bromide should be used with caution in patients with narrow-angle glaucoma, prostatic hypertrophy or bladder-neck obstruction. Patients should be advised that temporary blurring of vision, precipitation or worsening of narrow-angle glaucoma or eye pain may result if the solution comes into direct contact with the eyes. Use of a nebulizer with mouthpiece rather than face mask may be preferable, to reduce the likelihood of the nebulizer solution reaching the eyes. Patients should be advised that ipratropium bromide inhalation solution can be mixed in the nebulizer with salbutamol if used within one hour. Compatibility data are not currently available with other drugs. Patients should be reminded that ipratropium bromide Inhalation Solution should be used consistently as prescribed throughout the course of therapy.
Side Effects
Potentially life threatening effects - Idiosyncratic reactions to Ipratropium bromide are rare. Severe adverse effects due to inhibition of muscarinic receptors and ganglion blockade are theoretically possible but unlikely with the metered-dose aerosol. Severe/Irreversible adverse effects - No effect of this kind is reported. Symptomatic adverse effects - Regular use of Ipratropium can lead to a dry mouth through inhibition of salivary flow. Observed during clinical practice - The most common adverse reactions reported are -- dryness of the oropharynx (5%); cough, exacerbation of symptoms, & irritation from aerosol (3%); headache (2%); nausea, dizziness, blurred vision/difficulty in accommodation & drying of secretions (1%). Less frequently reported adverse reactions include tachycardia, nervousness, paresthesias, drowsiness, coordination difficulty, itching, hives, flushing, alopecia, constipation, tremor & mucosal ulceration. Case of precipitation or worsening of narrow-angle glaucoma, acute eye pain & hypotension have been reported.
Allergic-type reactions such as skin rash, angio-oedema of tongue, lips & face, urticaria (including giant urticaria), laryngospasm and anaphylactic reaction have been also reported; with positive rechallenge in some cases. Ipratropium bromide does not produce adverse effects on mucocilliary clearance, in contrast to atropine and other muscarinic antagonists. There is no evidence that in the therapeutic dose range Ipratropium has any adverse effect on bronchial secretion.
Pregnancy & Lactation
Pregnancy Category B. No adequate or well-controlled studies have been conducted in pregnant women. Because animal reproduction studies are not always predictive of human response, ipratropium bromide should be used during pregnancy only if clearly needed. It is not known whether ipratropium bromide is excreted in human milk.
Precautions & Warnings
Store at a cool & dry place, protected from light. Once a bottle has been opened the contents should be discarded after one month. Product should be used under the direction of a registered physician.
Storage Conditions
Should be put away in cool and dry put
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.