✔ 100% Authentic Product
👁️ Currently Viewing 4259
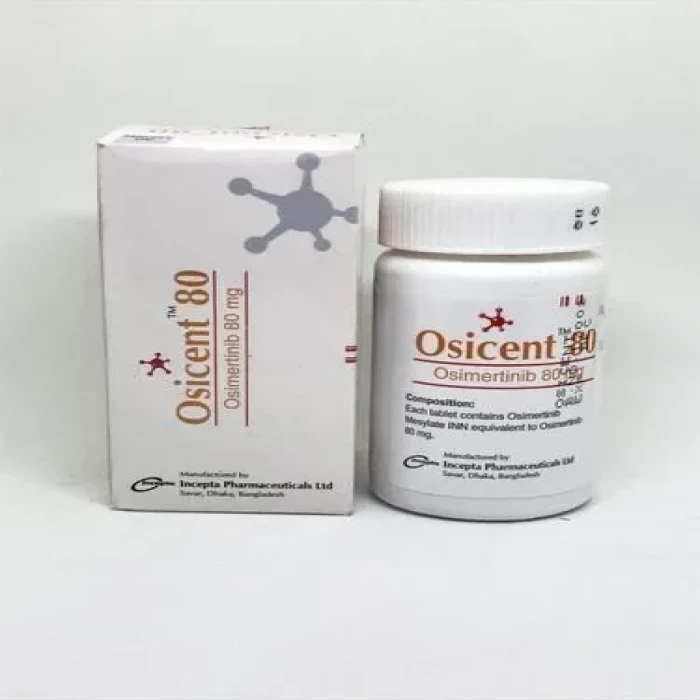
Osicent 80mg (30pcs Pot)
Osimertinib 80 belongs to a class of protein kinase inhibitors primarily used to manage or treat lung cancer.
Discount
Price: ৳ 14,700
MRP:
৳
15000
2%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Osicent 80 is a type of protein kinase inhibitor used to treat lung cancer. Lung cancer is a condition where cancerous cells grow uncontrollably in the lung tissues.
The active ingredient in Osicent 80, Osimertinib, works by blocking specific proteins in cancer cells that promote their growth, thereby slowing or stopping the progression of advanced cancer.
It is essential to take Osicent 80 as prescribed by your doctor for the recommended duration to achieve the best results. Common side effects include diarrhea, rash, dry skin, nail issues, stomatitis (oral inflammation), fatigue, reduced appetite, and low blood cell counts. If any of these side effects persist or worsen, consult your doctor.
Before starting Osicent 80, inform your doctor about any known allergies or hypersensitivity to medications or foods. This medication can harm a developing fetus, so it is crucial to avoid pregnancy during treatment and for several months afterward. Effective contraception is strongly recommended while undergoing therapy with Osicent 80. If you or your partner becomes pregnant during treatment, contact your doctor immediately. Breastfeeding is generally not recommended while on this medication. Additionally, it is advised to avoid alcohol during treatment. The safety and effectiveness of Osicent 80 in children have not been established.
✔️ Uses of Osicent 80
Treatment of Lung Cancer
✔️ How does Osicent 80 work?
Osicent 80 blocks proteins in cancer cells that encourage the cancer to grow. Osimertinib can temporarily reduce or stop the progression of advanced cancer. Following surgery, Osimertinib may help prevent the cancer from returning.
✔️ Side Effects of Osicent 80
The most common side effects (≥20%) include:
- Diarrhea
- Rash
- Dry skin
- Nail toxicity
- Stomatitis
- Fatigue
- Decreased appetite
✔️ Quick Suggestion For Pateint
- Include leafy vegetables, citrus fruits, fatty fish, berries, yogurt, apples, peaches, cauliflower, cabbage, broccoli, beans, and herbs.
- Monitor your blood pressure regularly while taking this medication. Inform your doctor if you notice symptoms of very high blood pressure such as severe headache, confusion, problems with your eyesight, nausea, or vomiting.
- Performing yoga may also help in improving both physical and mental health.
- Maintain a healthy weight by performing regular low-strain exercises and eating healthy food.
- Get optimal sleep; rest well.
- Avoid smoking and alcohol consumption.
- Avoid fast food, fried food, processed meats, refined carbs, and added sugars.
✔️ Indication of Osicent 80
- Osicent is approved for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations, as confirmed by an FDA-approved test.
- It is also indicated for patients with metastatic EGFR T790M mutation-positive NSCLC, confirmed by an FDA-approved test, whose disease has progressed following EGFR tyrosine kinase inhibitor (TKI) therapy.
Use under the guidance of a registered physician.
✔️ Pharmacology
Osimertinib, the active ingredient in Osicent, is an EGFR kinase inhibitor that binds irreversibly to specific EGFR mutations (T790M, L858R, exon 19 deletion) with significantly higher potency compared to wild-type EGFR.
- Activity: Exhibits anti-tumor effects in NSCLC with EGFR mutations and moderate inhibition of wild-type EGFR.
- Metabolites: Two active metabolites, AZ7550 and AZ5104, contribute to its activity, with AZ5104 showing higher potency against exon 19 deletions and T790M mutations.
- Pharmacokinetics: After oral dosing:
- Steady-state achieved after 15 days of daily dosing.
- Mean half-life: ~48 hours.
- Clearance (CL/F): 14.2 L/h.
- Absorption: Median time to peak plasma concentration (Cmax): 6 hours.
- Distribution: Volume of distribution (Vss/F): 986 L; plasma protein binding: 95%.
- Elimination: Excreted primarily in feces (68%) and urine (14%), with minimal unchanged drug excretion.
✔️ Dosage & Administration: Osicent 80
- 80 mg once daily until disease progression or unacceptable toxicity. May be taken with or without food.
- Skip the missed dose and take the next dose as scheduled.
- Disperse the tablet in 60 mL of non-carbonated water (do not crush or heat). Stir, drink immediately, and rinse the container with additional water (120–240 mL) to ensure the full dose is consumed.
- For nasogastric administration, disperse the tablet in 15 mL of water and use an additional 15 mL to rinse any residues. Administer promptly, followed by water flushes.
- Pediatric Use: Safety and efficacy not established.
- Geriatric Use: Older patients (≥65 years) may experience more severe side effects and dose modifications compared to younger patients.
- Renal Impairment: No dose adjustment is needed for mild, moderate, or severe impairment. Not studied in end-stage renal disease.
- Hepatic Impairment: No dose adjustments for mild to moderate impairment. Not studied in severe impairment.
✔️ Interaction
- CYP3A Inhibitors: Avoid strong CYP3A inhibitors (e.g., itraconazole, ritonavir) as they can increase Osicent plasma levels.
- CYP3A Inducers: Avoid strong inducers (e.g., rifampicin, St. John’s Wort) as they may decrease efficacy.
- Effect on Other Drugs: Osicent may alter plasma concentrations of drugs metabolized by CYP3A, BCRP, or CYP1A2, including fentanyl, cyclosporine, or quinidine. Monitor closely when used with these medications.
✔️ Contraindications:
None
✔️ Pregnancy & Lactation
- Pregnancy: May harm the fetus. Not recommended for use in pregnant women.
- Lactation: Unknown if Osicent is excreted in human milk; breastfeeding is not advised during treatment.
- Contraception:
- Females: Use effective contraception during treatment and for 6 weeks after the last dose.
- Males: Use effective contraception during treatment and for 4 months after the last dose.
- Fertility: May impair fertility in both males and females, though some effects on female fertility may be reversible.
✔️ Precautions & Warnings
- Interstitial Lung Disease (ILD)/Pneumonitis: Occurs in 3.9% of patients; 0.4% fatal. Discontinue if diagnosed.
- QTc Interval Prolongation: Monitor ECG and electrolytes in at-risk patients. Adjust dosage or discontinue as needed.
- Cardiomyopathy: Occurs in 1.4% of patients. Regularly assess left ventricular ejection fraction (LVEF).
- Embryo-Fetal Toxicity: Can cause fetal harm; advise proper contraception.
✔️ Storage:
Store below 30°C, away from light and moisture. Safely dispose of expired or unused medication. Keep out of reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.