✔ 100% Authentic Product
👁️ Currently Viewing 5016
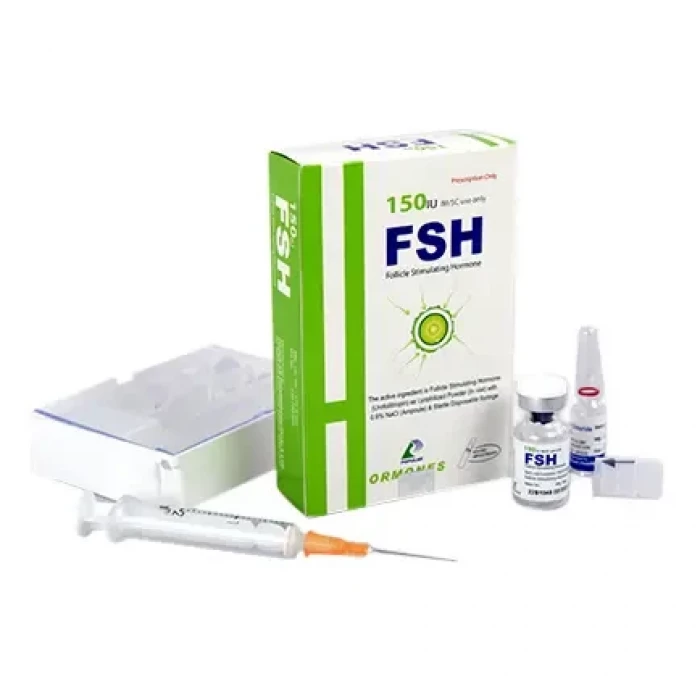
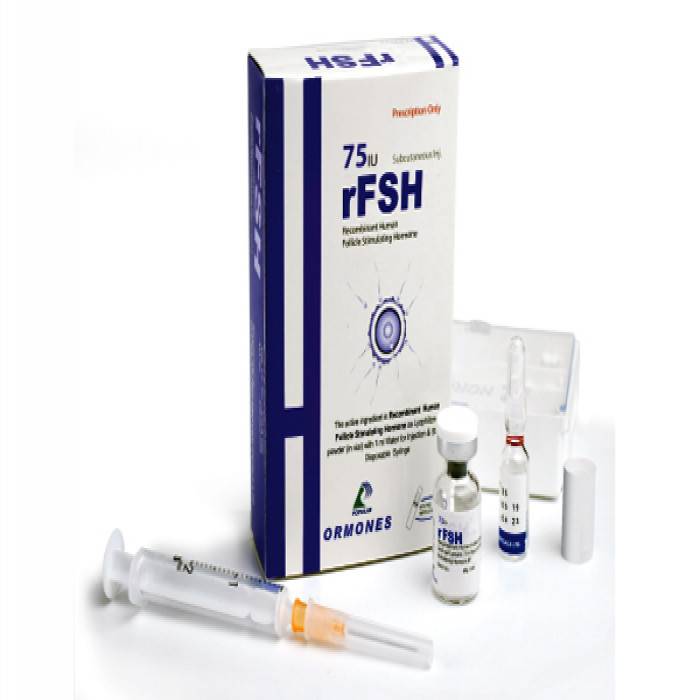
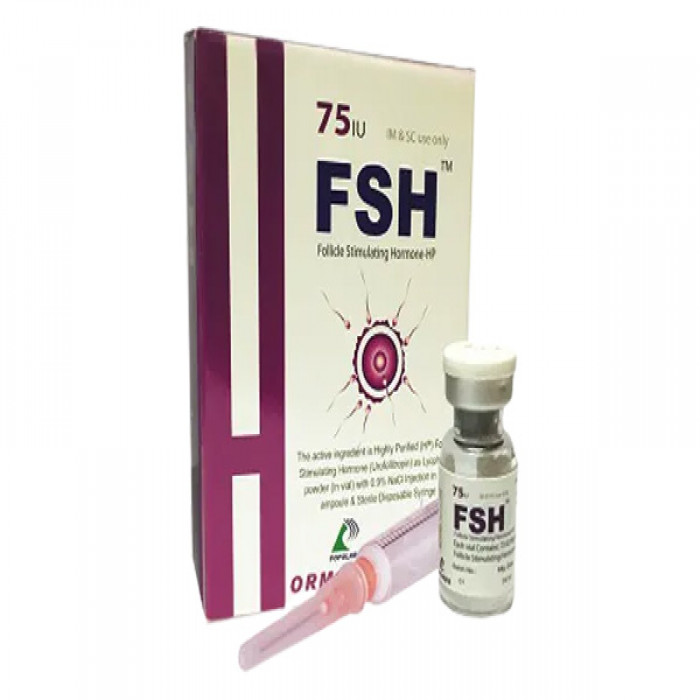
FSH 75IU IM/SC Injection
Follicle Stimulating Hormone
- Ovulation Induction
- Multi-follicular Development during Assisted Reproductive Technology (ART)
- Polycystic Ovarian Syndrome (PCOS) related infertility
- Male infertility treatment in combination with HCG for the induction of spermatogenesis
Discount
Price: ৳ 1,473
MRP:
৳
1550
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
FSH is a sterile, lyophilized powder intended for subcutaneous (SC) or intramuscular (IM) injection after reconstitution with sterile 0.9% Sodium Chloride Injection. It contains 75 IU & 150 IU of Follicle Stimulating Hormone (FSH) per vial.
Safety Advices

Alcohol
UNSAFE
It is unsafe to consume alcohol while using FSH 75IU IM/SC Injection.

Pregnancy
CONSULT YOUR DOCTOR
FSH 75IU IM/SC Injection is highly unsafe to use during pregnancy. Consult your doctor for advice, as studies on pregnant women and animals have shown significant harmful effects on the developing baby.
CONSULT YOUR DOCTOR
Information regarding the use of FSH 75IU IM/SC Injection during breastfeeding is not available. Please consult your doctor before using this medication while breastfeeding.

Driving
SAFE
FSH 75IU IM/SC Injection does not usually affect your ability to drive.

CONSULT YOUR DOCTOR
Limited information is available on the use of FSH 75IU IM/SC Injection in patients with kidney disease. Please consult your doctor before using this medication if you have kidney issues.

CONSULT YOUR DOCTOR
Limited information is available on the use of FSH 75IU IM/SC Injection in patients with liver disease. Please consult your doctor before using this medication if you have liver problems.
✔️ Uses of FSH 75IU IM/SC Injection
- Female Infertility
✔️ How does FSH 75IU IM/SC Injection work?
In females, FSH 75 IU stimulates the release of estrogen, aiding in egg development. It also helps develop more follicles (eggs) in women undergoing assisted reproductive technology procedures.
✔️ Side Effects of FSH 75IU IM/SC Injection
- Common side effects include headache, vaginal bleeding, nausea, and hot flashes.
- Serious side effects may include pelvic pain, breathing problems, and ovarian hyperstimulation syndrome (OHSS).
✔️ Quick Suggestions:
- Your doctor may prescribe additional medicines as part of a fertility treatment plan.
- Follow the recommended dosage and monitoring schedules to minimize the risk of ovarian hyperstimulation.
- Immediately inform your doctor if you experience severe pelvic pain, nausea, vomiting, diarrhea, sudden weight gain, trouble breathing, or decreased urination during treatment, as these could be symptoms of ovarian hyperstimulation syndrome (OHSS).
- Avoid using FSH 75IU IM/SC Injection if you are pregnant or breastfeeding, as it may increase the likelihood of multiple pregnancies.
- This medication stimulates egg production in women undergoing treatment for anovulatory infertility.
✔️ Indication of FSH 75IU IM/SC Injection
FSH 75 IU is primarily used in the treatment of female infertility. It helps stimulate the release of estrogen, promoting the growth and development of eggs before release. This medication is administered as an injection under the supervision of a doctor.
✔️ Pharmacology
FSH stimulates ovarian follicular growth in women who do not have primary ovarian failure. Treatment with FSH results in follicular growth and maturation, requiring HCG to induce ovulation.
There are no significant pharmacokinetic differences between intramuscular and subcutaneous administration of FSH. Both routes have an absolute bioavailability of approximately 77 percent.
✔️ Dosage of FSH 75IU IM/SC Injection
- Dosage varies depending on the individual's ovarian response and indication.
- Administration is typically subcutaneous or intramuscular.
- For ovulation induction, starting doses range from 50 to 150 IU, adjusted based on ovarian response.
- Detailed dosage instructions are provided for each indication.
Acute toxicity data in humans are not available. Overdose may lead to ovarian hyperstimulation syndrome.
✔️ Administration of FSH 75IU IM/SC Injection
Your doctor or nurse will administer this medication. Do not self-administer.
✔️ Interaction
No drug/drug interaction studies have been conducted for Urofollitropin in humans
✔️ Contraindications
- Tumors of the ovary, breast, uterus, pituitary, or hypothalamus
- Pregnancy or lactation
- Undiagnosed vaginal bleeding
- Hypersensitivity to the active substance or excipients
- Primary ovarian or testicular failure
✔️ Pregnancy & Lactation
FSH is contraindicated during pregnancy and lactation (Category X).
✔️ Precautions & Warnings
- Patients with non-gonadal endocrinopathies should be evaluated.
- Increased risk of multiple gestations (Multiple births) in pregnancies resulting from FSH treatment.
- Hypersensitivity reactions may occur.
- Ovarian hyperstimulation syndrome (OHSS) is a potential complication.
- Semen analysis is recommended in men undergoing treatment.
✔️ Storage Conditions:
- Store between 2°C to 8°C (in a refrigerator)
- Protect from light and moisture
- The reconstituted solution should be used immediately and any remaining solution discarded
✔️
vQ1:
Q2:
Q3:
Q4:
Q5:
Q6:
Q7:
Q8:
Q9:
Q10:
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.