✔ 100% Authentic Product
👁️ Currently Viewing 1335
Ribavirin is used in the treatment of chronic hepatitis C virus (HCV) infection. It is used in combination with interferon.
Discount
Price: ৳ 335
MRP:
৳
352.4
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
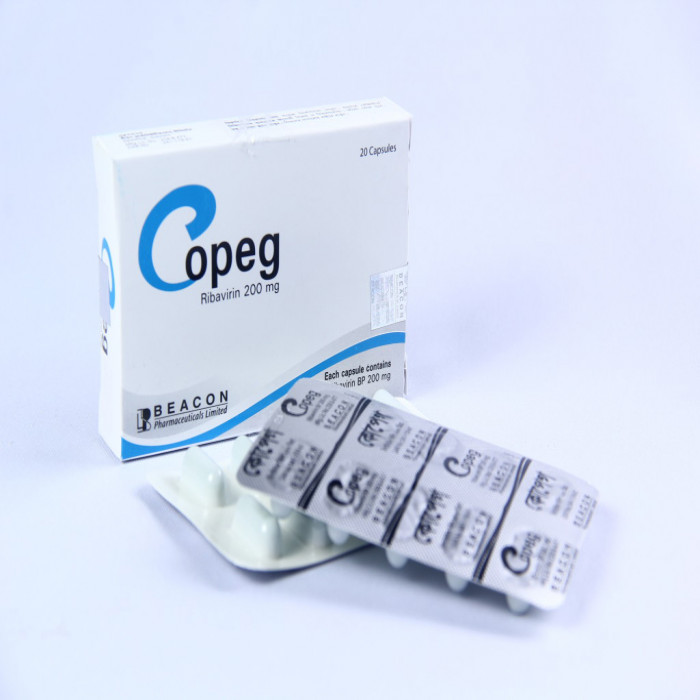
- Copeg 200mg Capsule is an antiviral medication used in the treatment of chronic hepatitis C virus (HCV) infection. It is taken in combination with other medicines for the treatment of this severe liver infection caused by the hepatitis C virus.
- The active ingredient in Copeg 200mg Capsule is Ribavirin, which is an antiviral drug. It works by inhibiting the action of viral RNA, which is essential for the virus to multiply. This helps reduce the amount of hepatitis C virus in the body and prevents it from making new viruses.
- To ensure proper treatment, it is crucial to take Copeg 200mg Capsule exactly as prescribed by your doctor and for the entire duration recommended based on your medical condition. Common side effects of the medication include dizziness, fever, headache, nausea, vomiting, tiredness, or loss of appetite. In most cases, these side effects are not serious and tend to resolve over time. However, if they persist or worsen, it is advisable to consult your doctor.
- If you are allergic to Copeg 200mg Capsule or any other medicines, it is essential to inform your doctor. The medication is not recommended for children below 3 years of age, and pregnant women should avoid taking it, as it may cause birth defects. If you are pregnant or breastfeeding, consult your doctor before using Copeg 200mg Capsule.
- To prevent pregnancy while using Copeg 200mg Capsule and for 7 months after stopping the treatment, it is recommended to use effective contraceptive measures. If you experience stomach pain, vomiting, or nausea while taking the medication, it could be a symptom of a rare but severe side effect called lactic acidosis, which requires immediate medical attention.
- Overall, it is important to follow your doctor's instructions and report any unusual or persistent side effects while using Copeg 200mg Capsule for the treatment of hepatitis C.
Safety Advices

Alcohol
UNSAFE
Consumption of alcohol is not recommended during treatment with Copeg 200mg Capsule.

Pregnancy
CONSULT YOUR DOCTOR
Copeg 200mg Capsule is not recommended for use in pregnant women unless necessary. Discuss the risks and benefits with your doctor.

Breastfeeding
CONSULT YOUR DOCTOR
Copeg 200mg Capsule is not recommended for use in breastfeeding women unless necessary. Discuss the risks and benefits with your doctor.

Driving
CAUTION
Copeg 200mg Capsule has very little effect on your ability to drive or use machines.

Kidney
CAUTION
Take Copeg 200mg Capsule with caution, especially if you have a history of Kidney diseases/conditions. The dose may be adjusted by your doctor as required.

Liver
CAUTION
Take Copeg 200mg Capsule with caution, especially if you have a history of Liver diseases/conditions. The dose may be adjusted by your doctor as required.
✔️ Uses of Copeg 200mg Capsule
- Chronic hepatitis C virus (HCV) infection
✔️ How does Copeg 200mg Cap work?sule
This medication is a ribonucleic analog that prevents viral RNA synthesis and viral mRNA clapping, thereby inhibiting nucleoside. In the form of purine RNA nucleotides, it affects RNA metabolism which is needed for viral replication.
✔️ Side Effects of Copeg 200mg Capsule
- Dizziness
- Fever
- Headache
- Nausea
- Vomiting
- Tiredness
- Loss of appetite
- Fatigue
- Anemia
- Hypotension
- Rash
- Conjunctivitis
- Bronchospasm
✔️ Quick Suggestions:
- Include plenty of vegetables and fruits: These are rich in vitamins, minerals, and antioxidants that support liver function and overall health.
- Choose whole grains: Opt for whole grains like brown rice, oats, quinoa, and barley, which provide essential nutrients and fiber.
- Consume lean protein: Include sources of lean protein such as skinless chicken, fish, beans, and egg whites, as they are beneficial for liver health.
- Incorporate healthy fats: Healthy fats found in avocados, olive oil, and nuts are important for the body and can support liver function.
- Use low-fat or non-fat dairy products: To minimize damage to the liver, choose low-fat or non-fat dairy options.
- Stay hydrated: Drink plenty of water to aid in the proper processing of food and support overall health.
- Limit sugary and high-salt foods: Avoid sugary foods like cakes, cookies, and sodas, as well as foods high in salt, as they may have negative effects on liver health.
- Avoid saturated fats: Stay away from foods containing saturated fats, such as fried foods, fatty cuts of meat, butter, and high-fat dairy products, as they can lead to weight gain and fatty liver.
- Avoid smoking and alcohol: Smoking and alcohol consumption can increase the risk of liver damage, so it is best to avoid them.
- Regular monitoring: While taking Copeg 200mg Capsule, it is essential to undergo regular blood tests to monitor blood, liver, and kidney functioning to ensure the medication is well-tolerated and effective.
✔️ Indication of Copeg 200mg Capsule
Ribavirin is an antiviral medication used to treat various viral infections, including hepatitis C, respiratory syncytial virus (RSV) infections, viral hemorrhagic fever, influenza A and B, and adenovirus infections. It is specifically indicated for severe lower respiratory tract RSV infections in patients with underlying compromising conditions such as prematurity, bronchopulmonary dysplasia, chronic lung conditions, congenital heart disease, immunodeficiency, immunosuppression, and recent transplant recipients.
✔️ Pharmacology
Ribavirin is a synthetic nucleoside that has inhibitory action against the respiratory syncytial virus, influenza virus, and herpes simplex virus. The mechanism of action is not clear. It may act at several sites including cellular enzymes to interfere with viral nucleic acid synthesis. The mono- and triphosphate derivatives are known to be responsible for the antiviral action of the compound.
✔️ Dosage & Administration of Copeg 200mg Capsule
The recommended duration of treatment for patients who have not been previously treated with interferon is 24 to 48 weeks. After 24 weeks of treatment, the virologic response should be assessed, and treatment discontinuation should be considered for patients who have not achieved an undetectable level of HCV RNA by that time. There are no safety and efficacy data on treatment for longer than 48 weeks in previously untreated patients.
For patients who have experienced a relapse following interferon therapy, the recommended duration of treatment is 24 weeks. There are no safety and efficacy data on treatment for longer than 24 weeks in patients who have experienced a relapse.
When using Ribavirin in combination with interferon or peg-interferon, the treatment duration and dosages depend on the HCV genotype and the patient's weight:
Ribavirin + Interferon:
- For Genotypes 1 and 4: (400 mg + 600 mg) ribavirin daily with 3 million international units (MIU) of interferon alpha-2a subcutaneously 3 times weekly for 48 weeks if the patient weighs less than 75 kg, and (600 mg + 600 mg) ribavirin daily for 24 weeks if the patient weighs more than 75 kg.
- For Genotypes 2 and 3: (400 mg + 400 mg) ribavirin daily with 3 million international units (MIU) of interferon alpha-2b subcutaneously for 24 weeks.
Ribavirin + Peg-Interferon:
- For Genotypes 1 and 4: (400 mg + 600 mg) ribavirin daily with 180 micrograms of peg-interferon alpha-2a subcutaneously once weekly for 48 weeks if the patient weighs less than 75 kg, and (600 mg + 600 mg) ribavirin daily for 48 weeks if the patient weighs more than 75 kg.
- For Genotypes 2 and 3: (400 mg + 400 mg) ribavirin daily with 180 micrograms of peg-interferon alpha-2b subcutaneously once weekly for 24 weeks.
Ribavirin can be taken with or without food, but it should be taken consistently. Drinking plenty of water while on treatment with Ribavirin is recommended to reduce the risk of serious side effects. Always follow your doctor's instructions and take Copeg 200mg Capsule as directed, swallowing the whole tablet without chewing, crushing, or breaking it.
✔️ Interaction
Drug-Drug Interaction: Copeg 200mg Capsule may interact with certain medications used to treat HIV/AIDS, such as stavudine, zidovudine, and didanosine. Additionally, it may interact with immunosuppressants like azathioprine.
Drug-Food Interaction: No interactions with food have been reported for Copeg 200mg Capsule.
Drug-Disease Interaction: Copeg 200mg Capsule should be avoided if you have any blood disorder or a history of a heart attack. Inform your doctor if you have conditions such as anemia, depression, severe eye disorders, dental disorders, HIV/AIDS, or heart problems before taking Copeg 200mg Capsule.
As with any medication, it is essential to inform your healthcare provider about all the medications you are taking, any medical conditions you have, and any allergies you may have to avoid potential interactions or adverse effects. Your doctor will determine the suitability of Copeg 200mg Capsule based on your individual health profile.
✔️ Contraindications
Ribavirin is contraindicated in pregnant women due to the potential risk of fetal harm. It should not be used by women who are pregnant or may become pregnant. If the drug is used during pregnancy or if the patient becomes pregnant while taking Ribavirin, the patient should be informed of the potential hazard to the fetus.
Additionally, Ribavirin should be used with caution in patients with hemoglobinopathies, such as thalassemia major or sickle-cell anemia. Combination therapy with Ribavirin and didanosine has been associated with reports of fatal hepatic failure, peripheral neuropathy, pancreatitis, and symptomatic hyperlactatemia/lactic acidosis in clinical trials.
As with any medication, it is crucial for healthcare providers to carefully consider the potential risks and benefits of Ribavirin treatment for each patient, taking into account their individual health conditions and medical history. Pregnant women or patients with certain pre-existing conditions may require alternative treatment options or close monitoring during Ribavirin therapy to minimize potential adverse effects.
✔️ Pregnancy & Lactation
Pregnancy Category X. Ribavirin produced significant embryocidal and/or teratogenic effects in all animal species in which adequate studies have been conducted. Malformations of the skull, palate, eye, jaw, limbs, skeleton, and gastrointestinal tract were noted. The incidence and severity of teratogenic effects increased with the escalation of the drug dose. Survival of fetuses and offspring was reduced.
Nursing Mothers: It is not known whether Ribavirin is excreted in human milk. Because many drugs are excreted in human milk and to avoid any potential for serious adverse reactions in nursing infants from ribavirin, a decision should be made either to discontinue nursing or therapy with Ribavirin, based on the importance of the therapy to the mother.
✔️ Precautions & Warnings
Ribavirin is associated with serious risks, particularly for pregnant women and their partners. It is essential to avoid using Ribavirin during pregnancy and for 6 months after treatment due to the potential for birth defects and fetal death. Prior to starting treatment, patients must have a negative pregnancy test, and both male and female patients must use at least two forms of contraception during treatment and for 7 months after treatment. This is to prevent pregnancy while on Ribavirin therapy.
- Special precautions should be taken for pediatric patients below the age of 3 years, as the safety and effectiveness of Ribavirin in combination with Peginterferon have not been established in this age group.
- For elderly patients with impaired renal function, the risk of toxic reactions to Ribavirin may be greater. The dose of Ribavirin should be reduced in patients with creatinine clearance less than or equal to 50 ml/min, and the dose of Interferon should be reduced in patients with creatinine clearance less than 30 ml/min.
- Patients who are allergic to Ribavirin or any other medicines should inform their doctor. Ribavirin is not recommended for children below 3 years of age and should be avoided in patients with any blood disorder or a history of a heart attack.
- Pregnant or breastfeeding women should consult a doctor before taking Ribavirin, and it is crucial to use effective contraceptive measures to prevent pregnancy during treatment and for 7 months after stopping Ribavirin therapy.
- Patients infected with the hepatitis C virus should take proper precautions to prevent the spread of the infection to others.
If any severe symptoms such as stomach pain, vomiting, or nausea occur while taking Ribavirin, immediate medical attention should be sought, as these could be signs of a rare but severe side effect known as lactic acidosis.
✔️ Storage Conditions
Store below 30°C. Keep protected from light. Keep medicines out of the reach of children. Do not use later than the date of expiry.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.