✔ 100% Authentic Product
👁️ Currently Viewing 2322
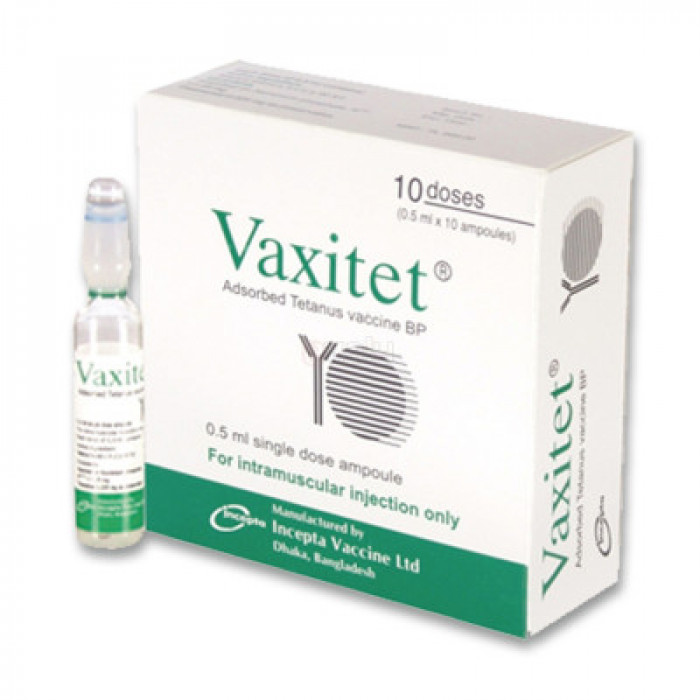
Vaxitet (0.5ml) Vaccine
Injection
Generic Name: Absorbed Tetanus Vaccine
Manufacturer: Incepta Pharmaceuticals Ltd.
Vaxitet : Each 0.5 ml dose contains > 5 Lf (potency > 40 IU) of tetanus toxoid adsorbed on aluminium phosphate equivalent to Al3+ < 1.25 mg.

100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City*
Regular Delivery - 12-24 Hours, Dhaka City* Regular Delivery - 24-48 Hours, All Over Bangladesh*
Regular Delivery - 24-48 Hours, All Over Bangladesh* ফ্রি ডেলিভারি! - ১৪৯৯ টাকা+ অর্ডারে ঢাকা
শহরে ।
ফ্রি ডেলিভারি! - ১৪৯৯ টাকা+ অর্ডারে ঢাকা
শহরে ।
✅ Description:
Description:
Vaxitet is a sterile suspension of tetanus toxoid adsorbed on aluminium phosphate suspended in an isotonic sodium chloride solution. The vaccine, after shaking, is a turbid liquid, whitish-gray in color. Adsorbed tetanus toxoid is prepared from tetanus toxin, produced by the growth of the bacterium Clostridium tetani in a peptone-based media. The toxin is converted to tetanus formol toxoid by treatment with formaldehyde solution. Formol tetanus toxoid is then purified, sterile, filtered and adsorbed to the aluminium phosphate.Thiomersal is added as preservative.
Indications:
• For the active immunization of infants, children 7 years of age or older and adults against tetanus, wherever combined antigen preparations are not indicated.
• For the prevention of neonatal tetanus in infants by immunizing women of childbearing age or infants born of unvaccinated pregnant women.
• Those who are liable to be exposed to tetanus infection and persons engaged in outdoor activities e.g. gardeners, agricultural, veterinary, athletes, industrial, sewage, road and outdoor workers, etc.
This vaccine is not to be used for the treatment of tetanus infection. If passive immunization is required, Tetanus Immune Globulin (TIG) (Human) should be used
Dosage and Administration:
Primary Immunization for Persons 7 Years of Age and Older:
A series of three doses of 0.5 ml each, of Adsorbed Tetanus vaccine should be given intramuscularly;
First dose: At appropriate date
Second dose: 4 to 8 weeks after the first dose
Third dose: 6 to 12 months after the second dose.
Children older than 7 years who did not complete primary immunization series (e.g., previously received only two doses of DTaP or DTP) need to receive only one dose of tetanus toxoid adsorbed vaccine to complete the primary series of tetanus.
Interruption of the recommended schedule with a delay between doses does not interfere with the final immunity achieved with Tetanus Toxoid Adsorbed vaccine. There is no need to start the series over again, regardless of the time elapsed between doses.
Routine Booster Injections:
To maintain adequate protection, a booster dose of 0.5 ml Adsorbed Tetanus vaccine every 10 years thereafter is recommended.
Vaccination of injured persons:
Clean and minor wound:
• If primary immunization confirmed and receiving booster dose within previous 5 yrs, no need of additional vaccine.
• If primary immunization confirmed and receiving booster dose more than previous 5 yrs, 1 dose of 0.5 ml.
All other dirty wounds (contaminated with feces, soil, and saliva):
• If primary immunization confirmed and receiving booster dose within previous 5 yrs, 1 dose of 0.5 ml.
• If primary immunization confirmed and receiving booster dose more than previous 5 yrs, 1 dose of 0.5 ml along with Tetanus immunoglobulin
If a person has no previous vaccination or uncertain, the primary series of 3 doses of 0.5ml adsorbed tetanus vaccine should be given along with Tetanus immunoglobulin with 1st dose.
Protection of neonatal tetanus:
• For prevention of neonatal tetanus, tetanus toxoid is recommended for immunization of women of childbearing age.
Women (15-49 Years): Vaccination Schedule
Number of doses Dose Interval between doses Administration
TT-1 0.5 ml At age of 15 years Intramuscular
TT-2 4 weeks after TT-1
TT-3 6 months after TT-2
TT-4 1 year after TT-3
TT-5 1 year after TT-4
• For pregnant woman who have not had previous immunization, at least 2 doses of tetanus toxoid at four weeks interval, 2 dose at least 2 weeks before delivery should be given during pregnancy so that protective antibody would be transferred to the infant in order to prevent neonatal tetanus.
Pregnant woman who have completed the course of tetanus, next 10 years no need of additional dose during pregnancy. Thereafter a single booster dose would be sufficient to extend immunity.
Method of Administration
Vaxitet is for intramuscular injection only. Do not inject intravenously. For adults and older children Vaxitet should be given intramuscularly in the deltoid muscle. For infants Vaxitet should be given intramuscularly in the anterolateral aspect of the upper thigh. It should not be injected into the gluteal areas as the immune response may be lower. The attending physician should determine final selection of the injection site and needle size, depending upon the patient's age and the size of the target muscle.
Preparation for administration
• The vaccine should be shaken well before use to obtain a homogenous turbid white suspension. Please do not shake vigorously.
• The vaccine should be inspected visually for particulate matter and discoloration prior to administration. If either of these conditions exist, the vaccine should not be administered.
• The vaccine should be used as supplied; no dilution is necessary.
• The full recommended dose of the vaccine should be used. Any vaccine remaining in a single-dose ampoule should be discarded.
Co – administration
Adsorbed tetanus vaccine can be given at the same time with other vaccine as diphtheria, tetanus, pertussis (DTP), polio (OPV), measles, mumps and rubella(MMR), Haemophilus Influenzae type b (Hib) and Meningococcal vaccines at separate sites with separate syringes. It should not be mixed with other vaccines or medicinal products in the same syringe.
Side Effects:
Adsorbed tetanus vaccine is generally well tolerated. Most recipients of tetanus vaccine experience some reactions upon vaccination. These are generally moderate and short in duration. They mainly consist of local reactions at the injection site (erythema, induration and tenderness). Systemic reactions (malaise and elevated temperature) are reported less commonly.
Contraindications:
Hypersensitivity to any component of the vaccine, including thiomersal, is a contraindication. This vaccine is contraindicated in patients with previous hypersensitivity to any tetanus-containing vaccine. Tetanus toxoid vaccination should be defferred during the course of any febrile illness or acute infection. A minor febrile illness such as a mild upper respiratory infection should not preclude immunization.
Use in Pregnancy and Lactation:
Pregnancy: For protection of neonatal tetanus, tetanus toxoid is recommended for immunization of women of childbearing age and especially pregnant women. Tetanus toxoid may be safely administered during pregnancy and should be given to the mother at first contact or as early as possible.
Lactation: it is not known if tetanus toxoid is excreted in human milk. It may be administered to nursing mothers only if clearly needed.
Over Dosage:
Not applicable.
Storage:
• Keep out of the reach and sight of children
• Store at +2 oC to +8 oC. Transportation should also be at +2 oC to +8 oC
• Do not freeze. Discard vaccine if frozen
• Protect from light
Commercial Pack:
Vaxitet : Each box contains 10 ampoules of Adsorbed Tetanus Vaccine of 0.5 ml each.
Vaxitet : Each box contains 1 vial of Adsorbed Tetanus Vaccine and a disposable syringe
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.