✔ 100% Authentic Product
👁️ Currently Viewing 4483
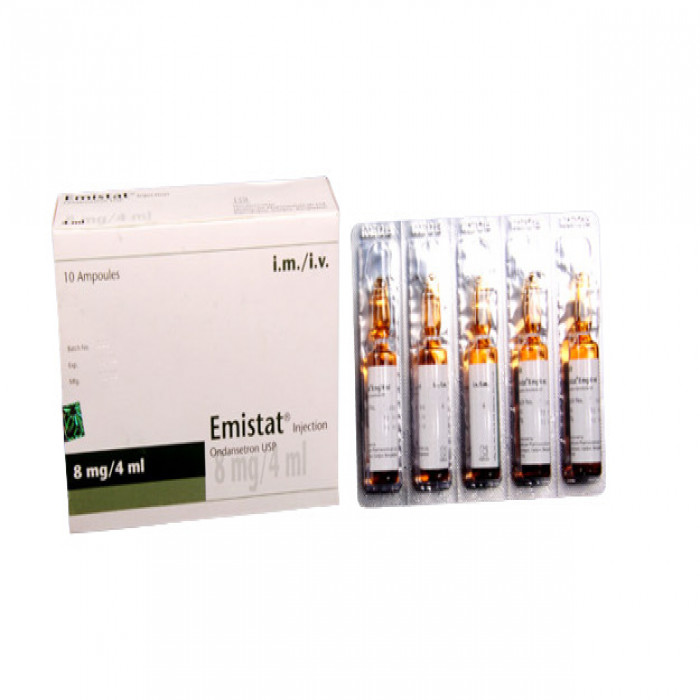
Emistat IM/IV-Injection (4ml) 1pc
Ondansetron is a medication that acts as a serotonin subtype 3 (5-HT3) receptor antagonist, and it is used for the following purposes:
- To prevent nausea and vomiting caused by initial and repeat courses of chemotherapy that cause nausea and vomiting (known as emetogenic cancer chemotherapy).
- To prevent and treat postoperative nausea and vomiting.
- To prevent nausea and vomiting induced by radiotherapy.
In summary, Ondansetron is used to manage nausea and vomiting caused by various medical treatments and procedures.
📄Prescription Required
Discount
Price: ৳ 30
MRP:
৳
32
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Emistat IM/IV-Injection is a medication that contains Ondansetron, a type of anti-emetic drug used to prevent and treat nausea and vomiting. It is particularly effective in cases of chemotherapy- or radiotherapy-induced nausea and vomiting, as well as postoperative nausea and vomiting.
However, before receiving Emistat IM/IV injection, it is important to inform your doctor if you have severe constipation, heart or liver problems, electrolyte imbalances, bowel obstruction, or if you have scheduled surgery.
Emistat IM/IV injection is not recommended for use during pregnancy, and caution should be exercised during breastfeeding since the medication may pass into breast milk. Dosage should be determined based on the patient's age, body weight, and medical condition.
While Emistat IM/IV injection is generally well-tolerated, some common side effects may include headaches, flushing, and constipation. If any of these symptoms persist or worsen, it is important to consult your doctor.
Safety Advices

Alcohol
CAUTION
The interaction of alcohol with Emistat IM/IV-Injection is unknown. Please consult a doctor before consuming alcohol with Emistat IM/IV-Injection.

Pregnancy
SAFE IF PRESCRIBED
If you are pregnant, inform your doctor before taking Emistat IM/IV-Injection. Your doctor will weigh the benefits and risks before prescribing this medicine.

Breastfeeding
CONSULT YOUR DOCTOR
Emistat IM/IV-Injection is probably safe to use during breastfeeding. Limited human data suggests that the drug does not represent any significant risk to the baby.

Driving
CAUTION
Emistat IM/IV-Injection usually does not affect your ability to drive or operate machinery.

Kidney
CAUTION
Emistat IM/IV-Injection may be safe to use in patients with kidney problems if prescribed by a doctor.

Liver
CAUTION
Take Emistat IM/IV-Injection with caution, especially if you have a history of Liver diseases/conditions. The dose may be adjusted by your doctor as required.
✔️ Uses of Emistat IM/IV-Injection
Used to treat and prevent nausea and vomiting induced by chemotherapy, radiotherapy, and surgery
✔️ How does Emistat IM/IV-Injection work?
Emistat IM/IV-Injection contains Ondansetron which blocks the action of serotonin, a chemical that stimulates the vomiting center (chemoreceptor trigger zone – CTZ) located in the brain. Thus, it prevents nausea and vomiting caused by chemotherapy in adults and children and also after surgery. Additionally, Emistat IM/IV-Injection can be used to prevent nausea and vomiting caused by radiotherapy for cancer in adults.
✔️ Side Effects of Emistat IM/IV-Injection
- Headache
- Flushing (feeling of warmth)
- Redness or irritation at the injection site
- Constipation
- Injection site pain
- Constipation
- Diarrhea
- Fatigue
- Uncontrolled body movements along with the upward rolling movement of the eyeballs
- Fits (seizures)
- Irregular heartbeat (fast or slow)
- Low blood pressure
- Hiccups
✔️ Quick Suggestions:
- You have been prescribed Emistat IM/IV-Injection for the prevention of nausea and vomiting caused after surgery or due to chemotherapy and radiotherapy.
- It is given as an injection into veins or as a drip under the supervision of a doctor.
- Avoid heavy meals and try eating small nourishing snacks throughout the day. Also, sip water regularly to help avoid dehydration.
- It may cause an application sites reaction like redness, swelling, and pain. Consult with your doctor if it bothers you.
✔️ Indication of Emistat IM/IV-Injection
Emistat IM/IV-Injection contains Ondansetron, which belongs to a group of medicines called Anti-emetics. It is used to treat and prevent nausea and vomiting induced by chemotherapy or radiotherapy. Emistat IM/IV-Injection Tablet is also used after surgeries (operations) to prevent nausea and vomiting.
✔️ Pharmacology
Ondansetron has the potential to be a potent and highly selective 5HT3 receptor antagonist. Its precise mechanism of action in the regulation of nausea and heaving is unknown. By activating vagal afferents via 5HT3 receptors, chemotherapeutic treatments, and radiation might elicit 5HT release inside the small intestinal tract, resulting in a spitting reflex. Ondansetron prevents the initiation of this response. The activation of vagal afferents may also result in a release of 5HT inside the zone postrema, which is located on the floor of the fourth ventricle, which may further promote emesis via a central component. As a result, the effect of ondansetron in the treatment of disease and vomiting caused by cytotoxic chemotherapy and radiation is most likely related to the antagonism of 5HT3 receptors on neurons present both in the peripheral and central nervous systems. The mechanisms of action in postoperative nausea and heaving are unknown, however, there may be shared pathways with cytotoxic-induced nausea and heaving.
✔️ Dosage & Administration of Emistat IM/IV-Injection
Chemotherapy-Induced Queasiness and Vomiting-
Adults, Pediatric patients (6 months to 18 years):
- Injection: Three 0.15 mg/kg dosages, up to a most extreme of 16 mg per dosage, imbued intravenously over 15 minutes.
Radiotherapy-Induced Sickness and Vomiting-
Adults:
- Injection: Three 0.15 mg/kg dosages, up to a greatest of 16 mg per dosage, implanted intravenously over 15 minutes.
Postoperative Queasiness and Vomiting-
Adults: Injection: 4 mg
It is important to note that these dosages are for informational purposes only and should not be used without the guidance of a healthcare professional. The appropriate dosage of Emistat IM/IV-Injection may vary depending on the patient's age, weight, medical condition, and other factors. Only a qualified healthcare professional can determine the correct dose and administration schedule for Emistat IM/IV-Injection in each individual case.
Your doctor or nurse will give you this Injection. Kindly do not self-administer.
✔️ Interaction
Emistat IM/IV-Injection may have interactions with various medications such as anticonvulsants, antituberculosis drugs, antibiotics, antifungal medications, narcotic analgesics, beta-blockers, anti-arrhythmic medicines, antidepressants, chemotherapy drugs, and antipsychotics. However, no significant drug-food interactions have been found for this injection.
If you have certain medical conditions such as gut blockages, liver problems, heart problems, or an imbalance of potassium, sodium, and magnesium in your blood, it's essential to inform your doctor before taking Emistat IM/IV-Injection. This information will help your doctor determine if this injection is safe for you and can adjust your treatment accordingly.
✔️ Contraindications
Contraindicated in patients known to have extreme touchiness to the medication or any of its components. Concomitant utilization of apomorphine.
✔️ Pregnancy & Lactation
Pregnancy Category B. Ondansetron is excreted within the breast milk of rats. It isn't known whether ondansetron is excreted in the human brain. Since numerous drugs are excreted in the human brain, caution ought to be worked out when ondansetron is managed by a nursing lady.
✔️ Precautions & Warnings
If you have a history of allergy to Ondansetron, Palanosetron, Granisetron, or any other medications, it's essential to inform your doctor before taking Emeset 2 Injection 2 ml. The injection should only be given to children in the recommended dosage based on their body weight or body surface area. If you're pregnant or breastfeeding, you should consult your doctor before using Emeset 2 Injection 2 ml. Additionally, if you have any issues with your blood's sodium, magnesium, or potassium salt levels, you should inform your doctor before using this injection.
✔️ Storage Conditions
Store at a temperature not surpassing 30ºC in a dry put. Secure from light and dampness.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.